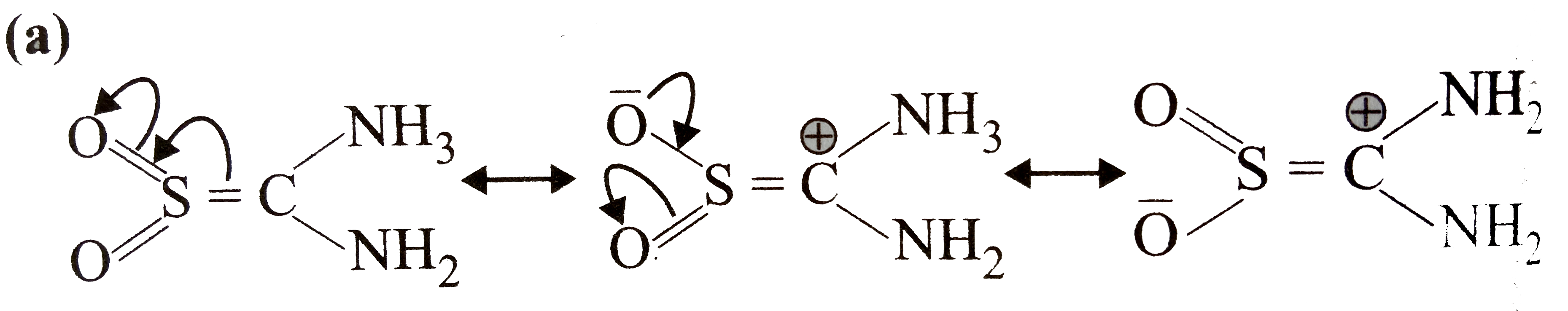
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Dipole Moment|28 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Valence Bond Theory (Vbt)|30 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosATOMIC STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Formal Charge, Resonance And Polarity Of Covalent Bond (Fajans Rule)
- Among the following the maximum convalent character is shown by the co...
Text Solution
|
- Which of the following has convant bond
Text Solution
|
- Polarization is the distortion of the anion by an adjacently placed ca...
Text Solution
|
- Maximum covalent character is associated with the compound
Text Solution
|
- Among LiCl, RbCl, BeCl(2) and MgCl(2) the compound with the greatest a...
Text Solution
|
- LiF a least soluble among the fluorides of alkali metals, because
Text Solution
|
- SaCI(4) is a convalent lquid because
Text Solution
|
- Which of the following Lewis structure does not contribute in resonanc...
Text Solution
|
- Carbonyl group has following resonating structures (I) The correc...
Text Solution
|
- Point out incorrect statement about resonance
Text Solution
|
- In compound O(2)SC(NH(2))(2). The geometry around the S, N and number ...
Text Solution
|
- Which of the following is leaser acceptable resonating atructure of N(...
Text Solution
|
- Which of the following pair constitute resonance structure?
Text Solution
|
- Which of the following statement about resonance energy is wrong?
Text Solution
|
- Aqueous solution of two compounds M(1) - O - H and M(2) - O - H are pr...
Text Solution
|
- Which of the following statement (s) is (are) true ? .
Text Solution
|
- In the anion HCOO^- , the carbon-oxygen bonds are found to be of equal...
Text Solution
|
- A metal, M from chaloride in its +2 and +4 oxidation states . Which of...
Text Solution
|
- The charge /size ratio of a cation determines its polarizing power. Wh...
Text Solution
|
- The correct statement for the molecule CsI(3) is
Text Solution
|