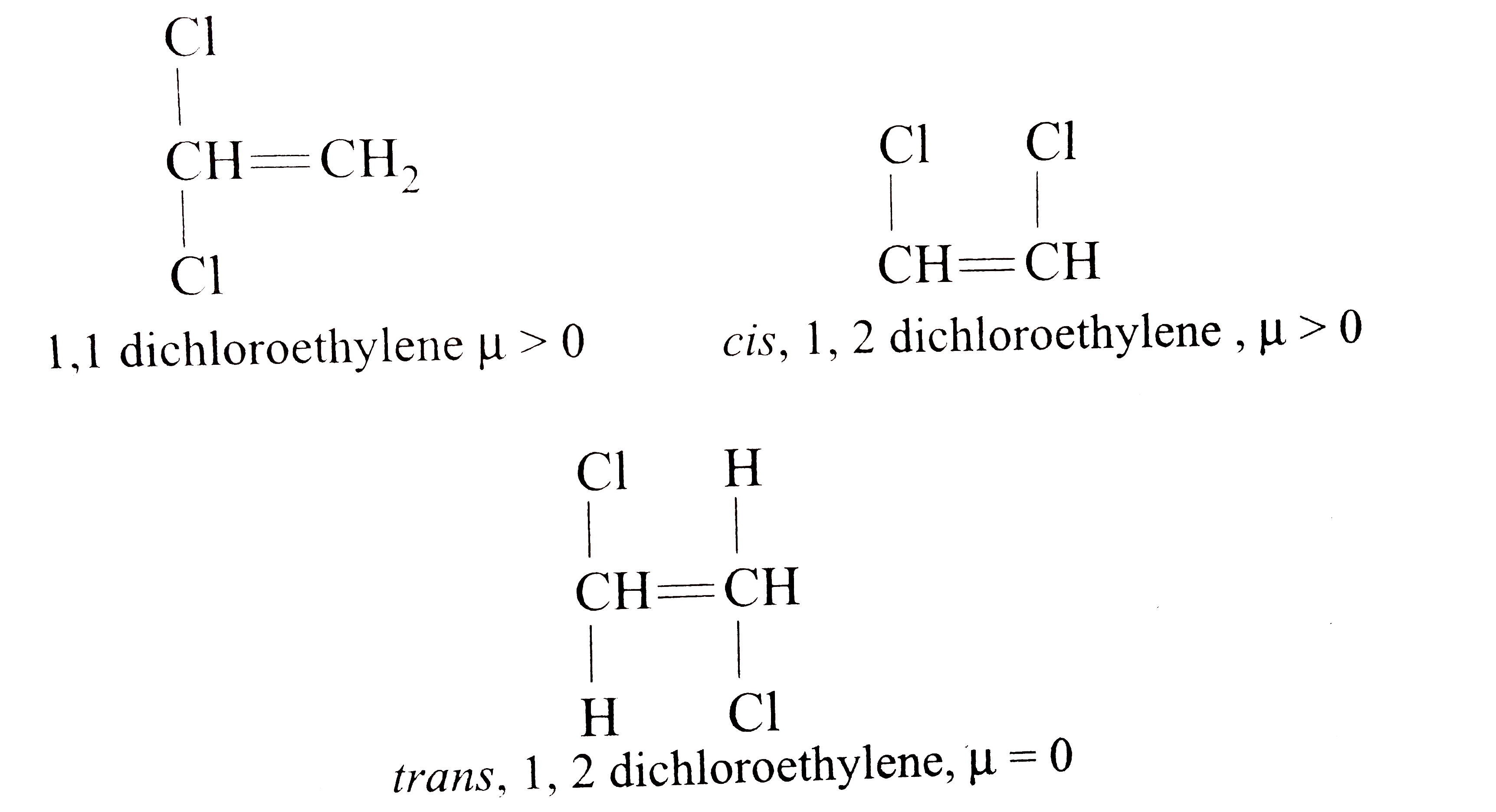
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Valence Bond Theory (Vbt)|30 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Vsepr Theory And Hybridisation|46 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Formal Charge, Resonance And Polarity Of Covalent Bond (Fajans Rule)|26 VideosATOMIC STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Dipole Moment
- For the formation of covalent bond, the different in the value of elec...
Text Solution
|
- Which of the following molecules will show dipole moment?
Text Solution
|
- Which bond angle theta would result in the maximum dipole moment for t...
Text Solution
|
- Carbon tetrachloride has no net dipole moment because of
Text Solution
|
- Pick out of molecule which has zero dipole moment?
Text Solution
|
- Which has maximum dipole moment?
Text Solution
|
- The dipole moment of HBr is 1.6 xx 10^(-30) cm and interatomic spaci...
Text Solution
|
- In a pole molecule , the ionic is 4.8 xx 10^(-10) esu. If the inter di...
Text Solution
|
- Which of the following will have zero dipole moment?
Text Solution
|
- BF(3) and NF(3) both molecules, are covalent, but BF(3) is non - polar...
Text Solution
|
- Which of the following is the correct order of dipole moment?
Text Solution
|
- Arrange the following compounds in order of increasing dipole moment ....
Text Solution
|
- Of the following molecules the one which has permaanent dipole moment ...
Text Solution
|
- Increasing order of dipole moment is
Text Solution
|
- Which contains both polar and non-polar bonds ? .
Text Solution
|
- The correct sequece of dipole moment among the chlorides of methane is
Text Solution
|
- The geometry of H2 S and its dipole moment are :
Text Solution
|
- Which of the following has been arrange in order of detereasing dipole...
Text Solution
|
- Which of the following has the least dipole moment?
Text Solution
|
- Which of the following molecules significant mu !=0?
Text Solution
|