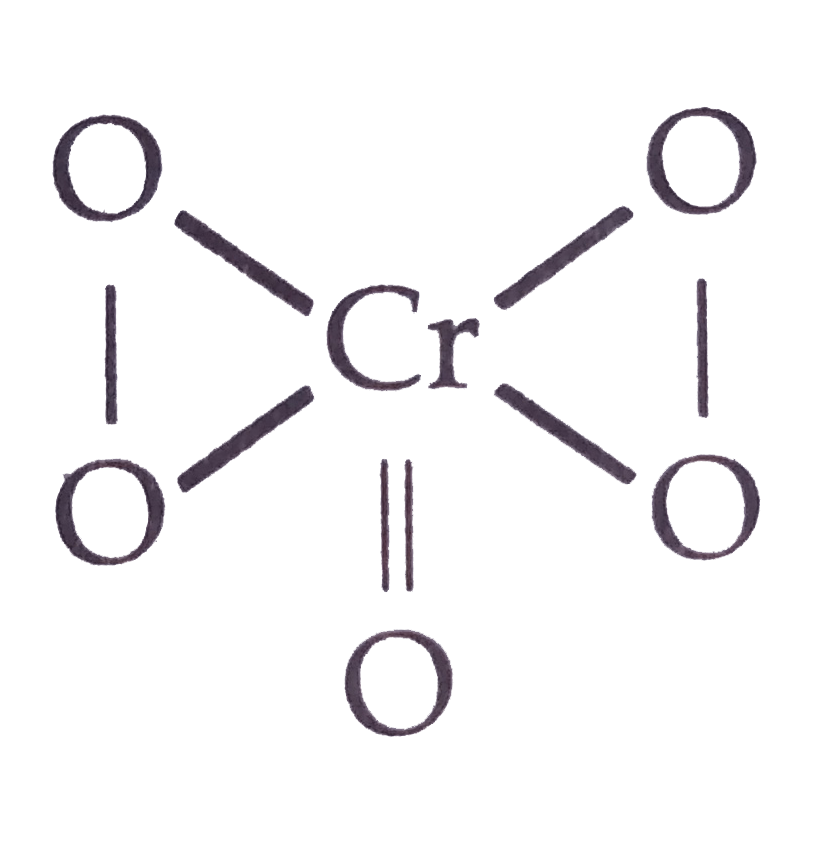
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-REDOX REACTIONS -TEST YOUR GRASP
- Oxidation state of chromium in
Text Solution
|
- When KMnO(4) reacts with acidified FeSO(4)
Text Solution
|
- Of the following reactions, only one is a redox reaction.Identify it.
Text Solution
|
- In which of the following pairs, there is greatest difference in the o...
Text Solution
|
- A,B and C are three elements forming part of a compound in oxidation s...
Text Solution
|
- When SO(2) is passed through the solution of potassium iodate,the oxid...
Text Solution
|
- In the reaction between SO(2) and SO(3) the equivalent weight of ozone...
Text Solution
|
- The atomic number of an element is 22. The highest oxidation state exh...
Text Solution
|
- The equivalent weight of potassium permanganate in acid solution is
Text Solution
|
- In the reaction which element undergoes oxidation as well as reduction...
Text Solution
|
- In the chemical reaction, K(2)Cr(2)O(7)+xH(2)SO(4)+ySO(2)rarrK(2)SO(...
Text Solution
|
- Consider the following reaction, 5H(2)O(2)+x ClO(2)+2OH^(-) to Cl^(-...
Text Solution
|
- xMnO4^(-) +yH2O2 rarr 2Mn^(2+) + 5 H2O + 9O2 + Ze^(-) In this reaction...
Text Solution
|
- The oxidation states of sulphur in the anions SO(3)^(2-), S(2)O(4)^(2-...
Text Solution
|
- 20 ml 0.18 M KOH is required to react with FeCl(2) to convert it into ...
Text Solution
|
- Of the following elements, which one has the same oxidation state in a...
Text Solution
|
- The oxidation number of carbon in CH2O is.
Text Solution
|
- The oxidation number of phosphorus in Ba(H(2)PO(2))(2) is:-
Text Solution
|
- When KMnO(4) is reduced with oxalic acid in acidic solution, the oxida...
Text Solution
|
- If three electrons are lost by a metal ion M^(3+), its final oxidation...
Text Solution
|
- Phosphorus has the oxidation state +3 in
Text Solution
|