A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-NATURE OF CHEMICAL BOND -TEST YOUR GRASP
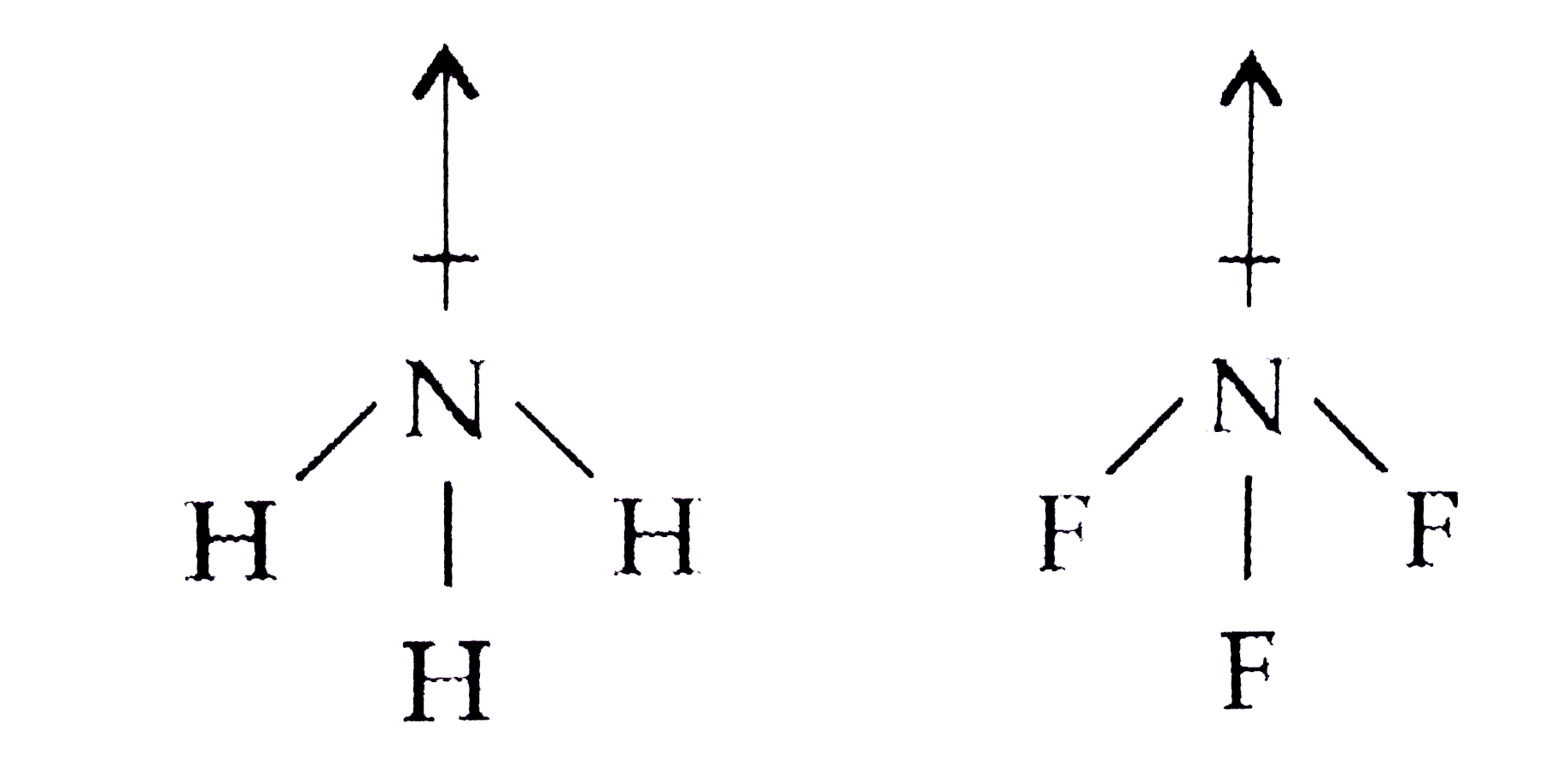
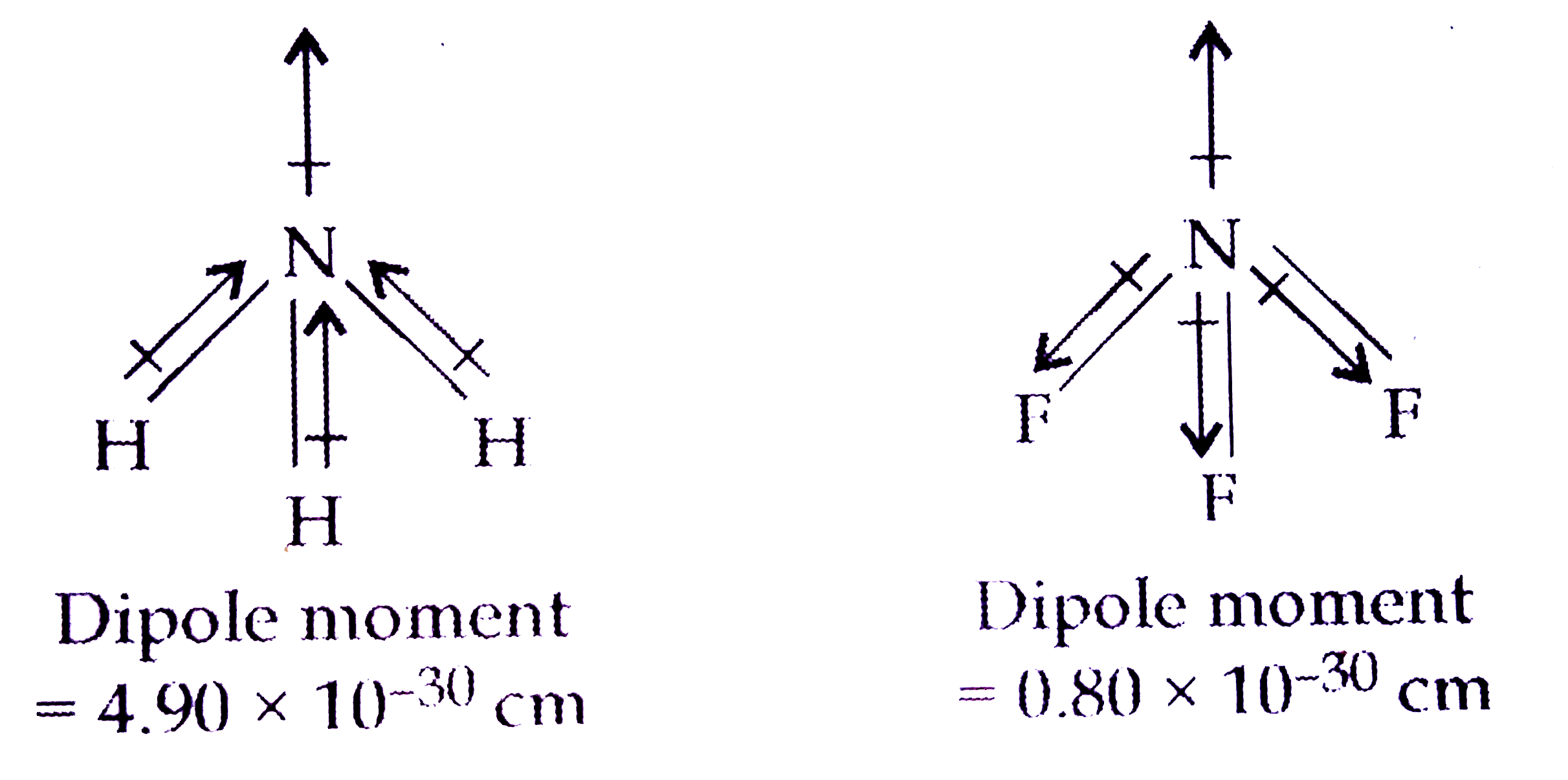
- Although F is more electronegative than H , then resultant dipole m...
Text Solution
|
- Which of the following does not follow the octet rule ? .
Text Solution
|
- The stability of ionic crystal depends principally on
Text Solution
|
- Which of the following statements about LiC and NaCl is wrong?
Text Solution
|
- Which of the following bonds is the strongest ? .
Text Solution
|
- Which of the following is the correct electron-dot structure of N2O mo...
Text Solution
|
- Which of the following molecule has highest dipole moment?
Text Solution
|
- Which of the following molecule has highest dipole moment?
Text Solution
|
- The correct order of decreasing polarity is
Text Solution
|
- In an ionic compound A^(+) X^(-) the degree of covalent bonding is gre...
Text Solution
|
- According to Fazan rule, the covalent bond is favoured by :
Text Solution
|
- Out of CHCl(3), CH(4) and SF(4) the molecules do not having regular ge...
Text Solution
|
- Which out of the following structures is expected to have three bond p...
Text Solution
|
- A molecule XY(2) contains two sigma bonds two pi bond and one lone pai...
Text Solution
|
- N atom in NH(4)^(+) involves the hybridizations
Text Solution
|
- A hybrid orbital formed from s and p-orbital can contribute to
Text Solution
|
- The state of hybridization of the central atom is not the same as in t...
Text Solution
|
- The d-orbitals involved in dsp^2 hybridisation is
Text Solution
|
- Which of the following molecules in planar ?
Text Solution
|
- The number of sp^2-s sigma bonds in benzene are
Text Solution
|
- The bond angle in H2O is nearly 105^@ whereas bond angle in H2 S is ne...
Text Solution
|