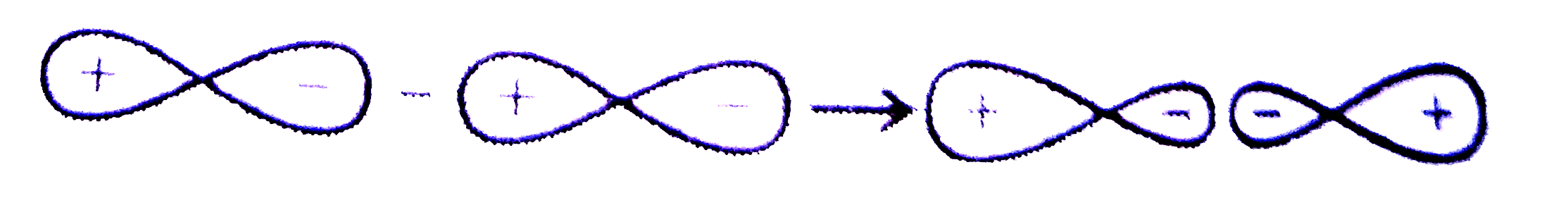
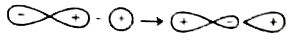A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
MARVEL PUBLICATION-NATURE OF CHEMICAL BOND -TEST YOUR GRASP
- Maximum number of H-bonds that can be formed by a water molecule is .
Text Solution
|
- Which of the following combination is not allowed in the LCAO method f...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- The calculated bond order of superoxide ion (O2^(-)) is
Text Solution
|
- The bond length of H(2)^(+), H(2)^(-) " and "H(2) are in the order
Text Solution
|
- Which of the following has unpaired electron in antibonding MO?
Text Solution
|
- Which species is paramagnetic in nature ?
Text Solution
|
- Mark the incorrect statement in the following :
Text Solution
|
- Lewis dot symbol of S (atomic no. 16) is
Text Solution
|
- Highest bond order among the following molecules C(2) , N(2), O(2...
Text Solution
|
- Maximum bond angle between two bond is in molecule.
Text Solution
|
- Total number of bonding molecular orbitals in O(2) molecule is
Text Solution
|
- Delta (delta ) molecular orbitals are formed by combination of
Text Solution
|
- Bond order of helium molecule is
Text Solution
|
- Among the following molecules PCl(5), SF(6), SCl(2), BeCl(2) , comple...
Text Solution
|
- Number of co-ordinate bond present in H(2) SO(4) molecule is
Text Solution
|
- C-C bond length is higest for
Text Solution
|
- Number of electrons around I in IF(7) is
Text Solution
|
- The structure of PF(5) molecule is .
Text Solution
|
- Bond angle in H(2)S molecule is
Text Solution
|