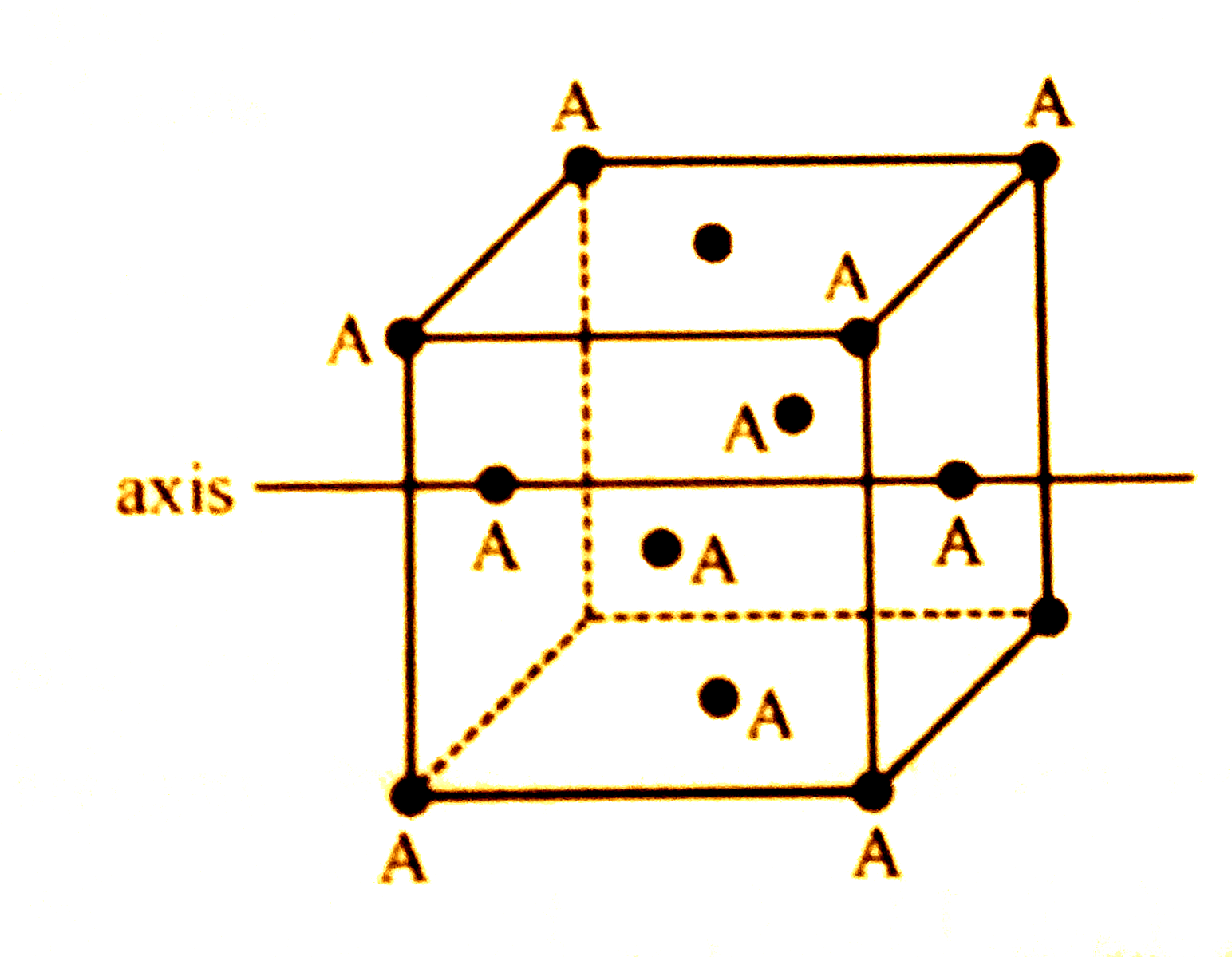
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-SOLID STATE-TEST YOUR GRASP
- In a solid AB having the NaCl structure, A atom occupies the corners o...
Text Solution
|
- The factor which makes a solid to have a low density is
Text Solution
|
- The close-packed layers in the faece-centred cubic unit cell are perpe...
Text Solution
|
- Bragg equation for the scattering of X-rays by crystal is
Text Solution
|
- Which of the following dimensions represent a hexagonal unit cell ?
Text Solution
|
- Which of the following lattices does not have only primitive Bravais l...
Text Solution
|
- Which of the following species is not paramagnetic?
Text Solution
|
- When electron are trapped into the crystal in anion cancy ,the defect ...
Text Solution
|
- The 8:8 type packing is present in
Text Solution
|
- The unit cell length of sodium chloride crystal is 360 pm. Its density...
Text Solution
|
- A substance which has face-centred cubic crystal has a density of 2.1...
Text Solution
|
- The fraction of volume occupied by atoms in a face centered cubic unit...
Text Solution
|
- The unit cell of an element having atomic mass 100 and density 12 g cm...
Text Solution
|
- A solid AB has NaCl structure. If the radius of the cation A is 100 pm...
Text Solution
|
- The phenomenon in which different chemical substances exhibit the same...
Text Solution
|
- In a close packed array of N spheres, the number of tetrahedral holes ...
Text Solution
|
- Solid iodine (I(2)) is an example of
Text Solution
|
- Which of the crystals should be the softest and have the lowest boilin...
Text Solution
|
- A semiconductor of Ge can be made p-type by adding
Text Solution
|
- What is the co-ordination number of each sphere in (i) Hexagonal clo...
Text Solution
|
- In a ferromagnetic material
Text Solution
|