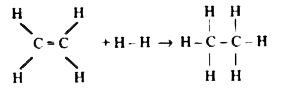A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS AND ENERGETICS
MARVEL PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (PREVIOUS YEARS ENTRANCE EXAMS)|76 VideosCHEMICAL THERMODYNAMICS AND ENERGETICS
MARVEL PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (TEST YOUR GRASP)|50 VideosCHEMICAL THERMODYNAMICS AND ENERGETICS
MARVEL PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (Third Law of Thermodynamics)|6 VideosCHEMICAL KINETICS
MARVEL PUBLICATION|Exercise TEST YOUR GRASP|50 VideosCHEMISTRIY IN EVERYDAY LIFE
MARVEL PUBLICATION|Exercise TEST YOUR GRAPS|24 Videos
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-CHEMICAL THERMODYNAMICS AND ENERGETICS-MULTIPLE CHOICE QUESTIONS (HIGHER LEVEL)
- If the bond dissociation energies of XY,X(2) and Y(2)( all diatomic mo...
Text Solution
|
- In conversion of lime-stone to lime, CaCO(3(s)) to CaO((s)) + CO(2(g...
Text Solution
|
- If a 298K the bond energies of C-H,C-C,C=C and H-H bonds are respectiv...
Text Solution
|
- Standard entropy of X(2), Y(2) and XY(3), Delta H =-30 kJ. To be at ...
Text Solution
|
- For a particular reversible reaciton at temperature T, DeltaH and Delt...
Text Solution
|
- Choose the correct option about the following sentences. [T - True, F ...
Text Solution
|
- Match the following :
Text Solution
|
- For the reaction, bond energies are given as under (i) C-C, 346 k...
Text Solution
|
- Heat of vapourisation of benzene is 7350 calorie K^(-1) mol^(-1). Calc...
Text Solution
|
- Calculate equilibrium constant for a reaction 2Br^(-1)+I(2) hArr 2I^...
Text Solution
|
- Which one of the following is not a characteristic of thermodynamics ?
Text Solution
|
- For the reaction N(2)O(4(g)) rarr 2NO(2(g)) , Delta H^(@) =57.24 kJ ...
Text Solution
|
- The temperature at which change over between spontaneous and non-spont...
Text Solution
|
- By measuring heat capacity of the solid at various temperature can be...
Text Solution
|
- If an endothermic reaction is non-spontaneous at freezing point of wat...
Text Solution
|
- The internal energy change when a system goes fromk state A to B is 40...
Text Solution
|
- If a 298K the bond energies of C-H,C-C,C=C and H-H bonds are respectiv...
Text Solution
|
- In an irreversible process taking place at constant T and P and in whi...
Text Solution
|
- The enthalpies of combustion of carbon and carbon monoxide are -393.5 ...
Text Solution
|
- The exothermic formation of ClF(3) is represented by thr equation: C...
Text Solution
|