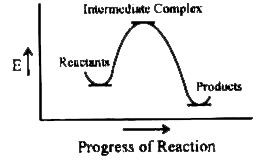
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS AND ENERGETICS
MARVEL PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (PREVIOUS YEARS ENTRANCE EXAMS)|76 VideosCHEMICAL THERMODYNAMICS AND ENERGETICS
MARVEL PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (TEST YOUR GRASP)|50 VideosCHEMICAL THERMODYNAMICS AND ENERGETICS
MARVEL PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (Third Law of Thermodynamics)|6 VideosCHEMICAL KINETICS
MARVEL PUBLICATION|Exercise TEST YOUR GRASP|50 VideosCHEMISTRIY IN EVERYDAY LIFE
MARVEL PUBLICATION|Exercise TEST YOUR GRAPS|24 Videos
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-CHEMICAL THERMODYNAMICS AND ENERGETICS-MULTIPLE CHOICE QUESTIONS (HIGHER LEVEL)
- The entropy change involved in the isothermal reversible expansion of ...
Text Solution
|
- The incorrect expression among the following is
Text Solution
|
- For an endothermic reaction, where Delta H represents the enthalpy of ...
Text Solution
|
- Molar heat capacity of water in equilibrium with ice at constant press...
Text Solution
|
- The DeltaH(f)^(@) for CO(2)(g), CO(g) and H(2)O(g) are -395.5,-110.5 a...
Text Solution
|
- Which one of the following statements is false?
Text Solution
|
- Two moles of an ideal gas is expanded isothermally and reversibly from...
Text Solution
|
- The enthalpy of vaporisation of a liquid is 30 kJ mol^(-1) and entropy...
Text Solution
|
- The value of log(10)K for a reaction A hArr B is (Given: Delta(f)H(298...
Text Solution
|
- The entropy change can be calculated by using the expression DeltaS-(q...
Text Solution
|
- Which of the following is not a correct statement about enthalpy of so...
Text Solution
|
- If enthalpy of an overall reaction X gt Y along one route is Delta(r)H...
Text Solution
|
- For a reaction, CaCO(3(s)) rarr CaO((s))+CO(2(g)) Delta (f) H^(0) (C...
Text Solution
|
- What will be the heat of reaction for the following reaction? Will the...
Text Solution
|
- A system changes from state X to Y with a change in internal energy me...
Text Solution
|
- At dynamic equilibrium the reaction on both sides occur at the same ra...
Text Solution
|
- 50 mL of 1 M NaOH is mixed with 50 mL of 1 M HCI and rise in temperatu...
Text Solution
|
- Read the following statements regarding spontaneity of a process and m...
Text Solution
|
- Work done on an ideal gas in a cylinder when it is compressed by an ex...
Text Solution
|
- When 50 mL of a strong acid is added to 50 mL of a strong base tempera...
Text Solution
|