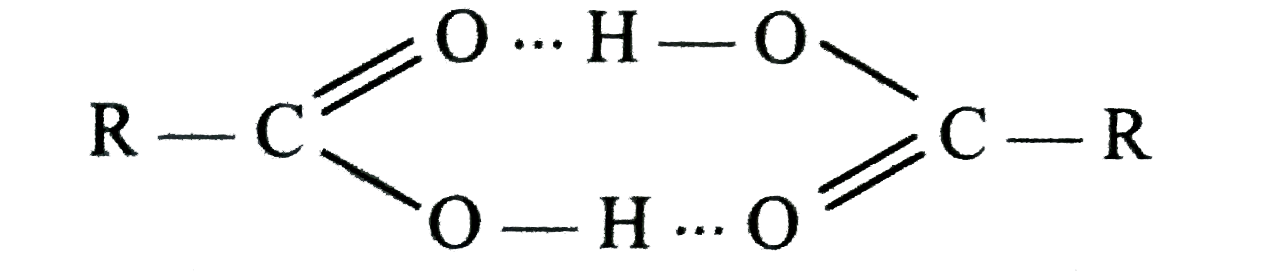A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACID
A2Z|Exercise AIPMT/NEET Questions|43 VideosALDEHYDES, KETONES AND CARBOXYLIC ACID
A2Z|Exercise AIIMS Questions|40 VideosALDEHYDES, KETONES AND CARBOXYLIC ACID
A2Z|Exercise Section A (Topicwise question|3 VideosALCOHOLS, PHENOLS AND ETHERS
A2Z|Exercise Section D - Chapter End Test|30 VideosBIOMOLECULES
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ALDEHYDES, KETONES AND CARBOXYLIC ACID-Section B - Assertion Reasoning
- Assertion: Lower aldehydes and ketonses are solutble in water but the ...
Text Solution
|
- Assertion: Formic acid reduces mercuric chloride to mercurous chloride...
Text Solution
|
- Assertion: Boiling and melting point of amides are higher than corresp...
Text Solution
|
- Assertion: Hydroxyketones are not directly used in Grignard reaction. ...
Text Solution
|
- Assertion: Isobutanal does not give iodoform test. Reason : It does ...
Text Solution
|
- Assertion: The pK(a) of acetic acid is lower than that of phenol. R...
Text Solution
|
- Assertion: Acetamide has more polargt C = 0 group than ethyl acetoacet...
Text Solution
|
- Assertion: (CH(3))(3)C COC(CH(3))(3) and acetone can be distanguished ...
Text Solution
|
- Assertion: Cyclohexanone exhibits keto-enol tautomerism. Reason : In...
Text Solution
|
- Assertion: Benzaldehyde is more reactive than ethanal towards nucleoph...
Text Solution
|
- Assertion: p-N, N-dimethylaminobenzaldehy under-goes benzoin condensat...
Text Solution
|
- Assertion: In sodium formate, both the C - O bonds have same value 1.2...
Text Solution
|
- Assertion: Esters which contain alpha-hydrogens undergo Claisen conden...
Text Solution
|
- Assertion: beta-Keto carboxylic acids lose CO(2) when heated of about ...
Text Solution
|
- Assertion: The acetate ion is resonance stablized Reason : Acetate i...
Text Solution
|
- Assertion: p-hydroxybenzoic acid has a lower boiling point than o-hydr...
Text Solution
|
- Assertion: Aromatic aldehydes can be distinguished from aliphatic alde...
Text Solution
|
- Assertion: Paracids are stronger acids than corresponding carboxylic a...
Text Solution
|
- Assertion: Acerylic acid (CH(2) - CHCOOH) is a weaker acid than benzon...
Text Solution
|
- Assertion: Acetoacetic ester (CH(3)COCH(2)COOHH(5)) contains CH(3)CO g...
Text Solution
|