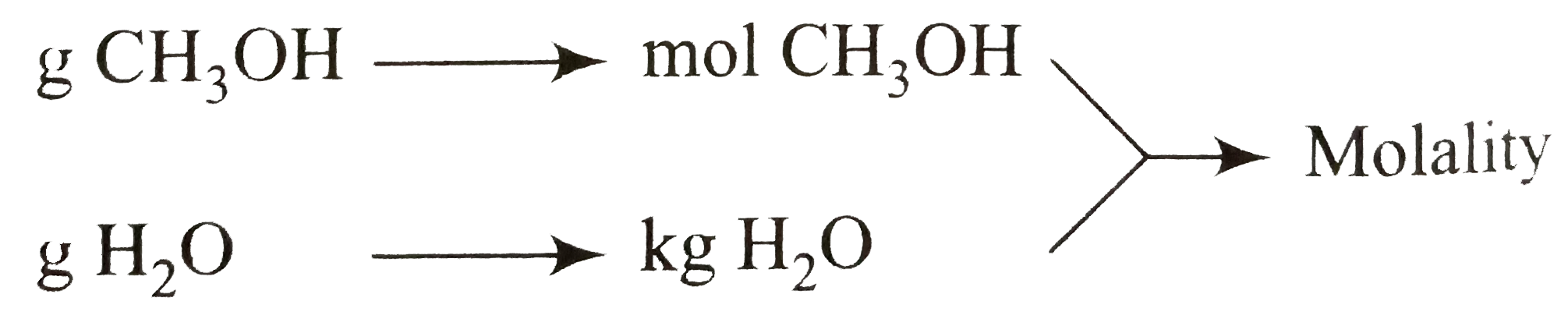Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-SOME BASIC CONCEPTS OF CHEMISTRY-Archives
- Molarlity: An acqueous solution contains 128g of mehanol (CH(2) OH) i...
Text Solution
|
- 6.02xx10^(20) molecules of urea are present in 100 mL solution. The co...
Text Solution
|
- 100 mL of phosphine (PH(3)) on hearing forms phosphorous (P) and hydr...
Text Solution
|
- Common salt obtained from sea water contains 95% NaCl by mass. The app...
Text Solution
|
- 10g of hydregoen and 64g of oxygen were filled in a steel veasel and ...
Text Solution
|
- How many moles of lead (II) chloride will be formed from a reaction be...
Text Solution
|
- What volume of oxygen gas (O(2)) measured of 0^(@)C and 1 am needed t...
Text Solution
|
- An element X has the following istopic compositon: .^(200)X(90%), ...
Text Solution
|
- Number of atoms of He in 100 atoms of He (at.mass 4 amu) is
Text Solution
|
- Which among the following is the heavist?
Text Solution
|
- The mass of carbon anode consumed (giving only carbon dioxide) in the ...
Text Solution
|
- What is the equivalent weight of phosphoric acid (H(3) PO(4)) accordin...
Text Solution
|
- 18 carat gold contains
Text Solution
|
- Number of water molecules in a drop of water, if 1 mL of water has 20 ...
Text Solution
|
- The maximum number of molecules is present in
Text Solution
|
- What will be the volume of the mixture after the reaction
Text Solution
|
- In Haber process 30 litre of dihydrogen and 30 litres of dinitrogen we...
Text Solution
|
- A compound has hemoglobin-like structure. It has one Fe and contains ...
Text Solution
|
- Among (i) FeSO(4). 7H(2)O, (ii) CuSO(4). 5H(2)O, (iii) ZnSO(4). 7H(2)O...
Text Solution
|
- If oxygen is present in 1 L flask at a pressure of 7.6xx10^(-10) mm Hg...
Text Solution
|
- In the reaction Zn(s) + 2H^(+)(aq.) rarr Zn^(2+)(aq.) + H(2) (g), how ...
Text Solution
|