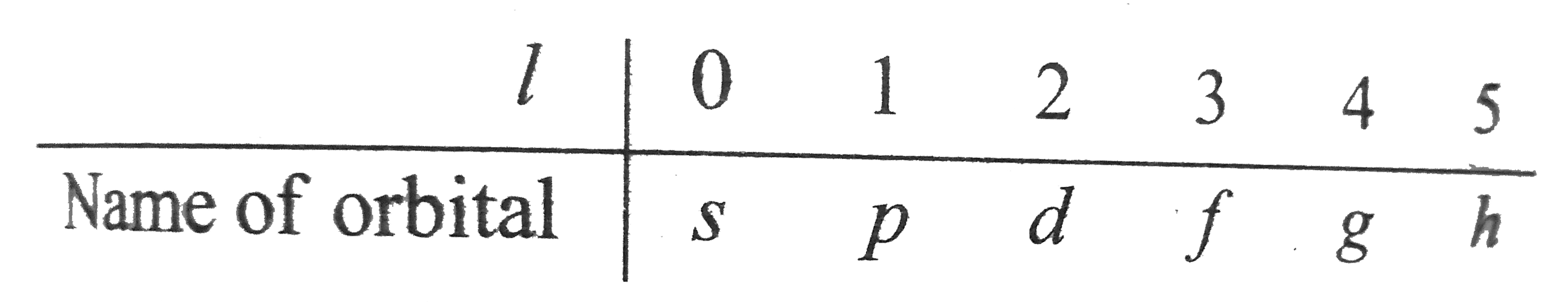A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-STRUCTURE OF ATOM-Follow-up Test
- The angular momentum quantum number (l) does not tell us theof the orb...
Text Solution
|
- The possible values of l depend on the value of the principal quantum ...
Text Solution
|
- The value of lis generally designated by the eletters s,p,d…. Thus, if...
Text Solution
|
- magnetic quantum number, m(l), distingushes the orbitals of given n an...
Text Solution
|
- The allowed values of magnetic quantum nunber (m(l)) are the integers ...
Text Solution
|
- There areorbitals in each subshell o fthe quantum number l.
Text Solution
|
- How many orbitals are possible for n = 2 and l = 1?
Text Solution
|
- Which of the following is incorrect?
Text Solution
|
- Spin quantum number (m(s)) refers to thepssible orientations of the sp...
Text Solution
|
- Experiements on the emission spectra ofatoms gave rise to a fourth qua...
Text Solution
|
- Conclusive proof of electron spin was provided by O. Strem and W. Gerl...
Text Solution
|
- Which of the following is incorrect?
Text Solution
|
- Which of the following sate of quantum numbers is not permissible for ...
Text Solution
|
- Which of the following combinations of quantum numbers is possible for...
Text Solution
|
- Strictly specking, an orbital does not have a well-defined shape becau...
Text Solution
|
- For which of the following orbitals is the radial probaility density R...
Text Solution
|
- ns orbital hasradial nodes.
Text Solution
|
- How many peacks are present in the radial distribution function for th...
Text Solution
|
- The radius of maximum probability for 1s orbital of H atom is
Text Solution
|
- All s orbitals are spherically symmetrical, meating that the probabili...
Text Solution
|