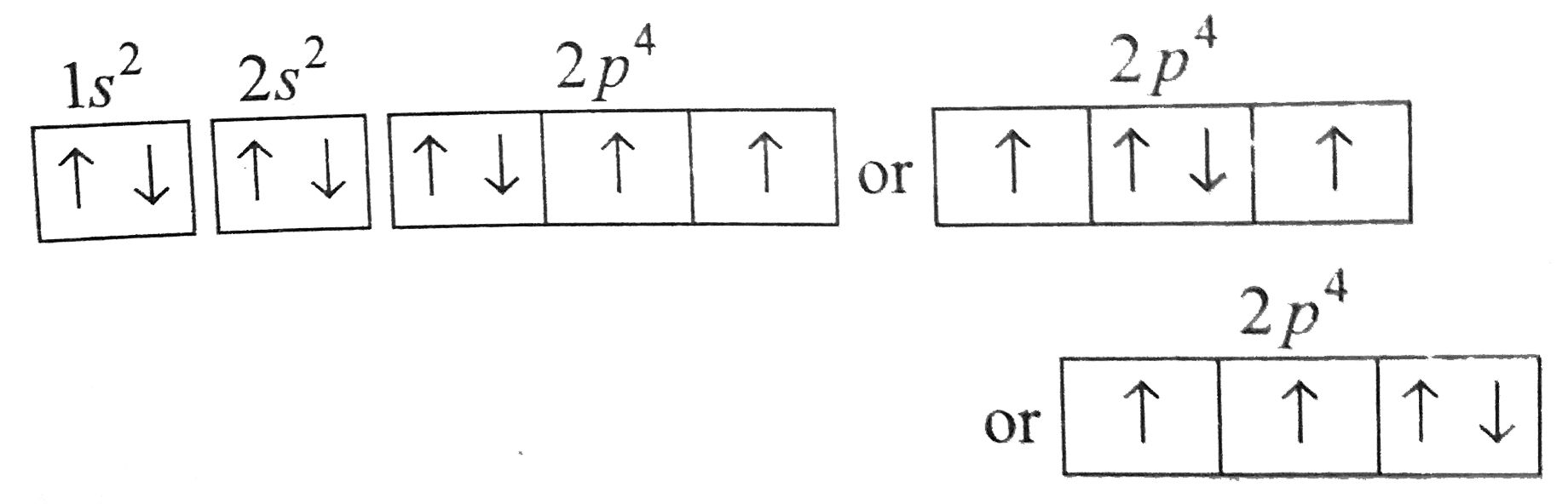
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-STRUCTURE OF ATOM-Follow-up Test
- All s orbitals are spherically symmetrical, meating that the probabili...
Text Solution
|
- How many nodal planes pass through the nucleus for a g orbital?
Text Solution
|
- In case of p(z) orbital, theis a nodal plane.
Text Solution
|
- Which of the following d orbitals has diagonal nodal planes?
Text Solution
|
- Which of the following orbitals is called the ground state of an H ato...
Text Solution
|
- Which of the following orbitals has maximum enegry?
Text Solution
|
- Which of the following electrons extres the maximum shileding effect i...
Text Solution
|
- Which of the following electrons possesses the minimu penetrating powe...
Text Solution
|
- The electrons identified by the following quantum numbers n and l: (i)...
Text Solution
|
- What is the maximum number of electrons that can be placed in 4f(xyz) ...
Text Solution
|
- What is the maximum number of electrons that can be placed in each she...
Text Solution
|
- What is the maximum number of electrons that can be placed in each sub...
Text Solution
|
- The orbital diagram in which the Aufbau principle is violated is
Text Solution
|
- Which of the following is a violation of the Pauli exclusion principle...
Text Solution
|
- Which of the following is a violation of the Hund's rule?
Text Solution
|
- Which of the following is the right electronic confiuration of the ele...
Text Solution
|
- How many unpaired electrons are present in the atomic mercury (Z = 80)...
Text Solution
|
- An oxygen atom has a total of eight electrons. The correct set of four...
Text Solution
|
- The extra stability of half-filled and completely-filled subshell is d...
Text Solution
|
- Which of the following metaks has the highest value of exchnage enegry...
Text Solution
|