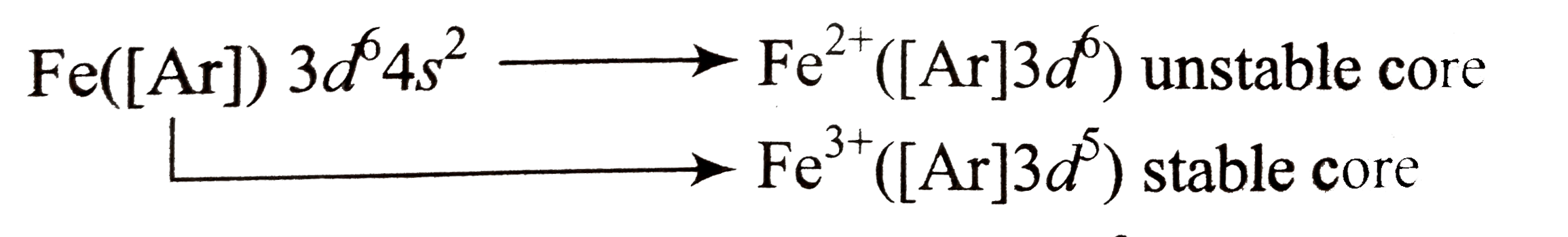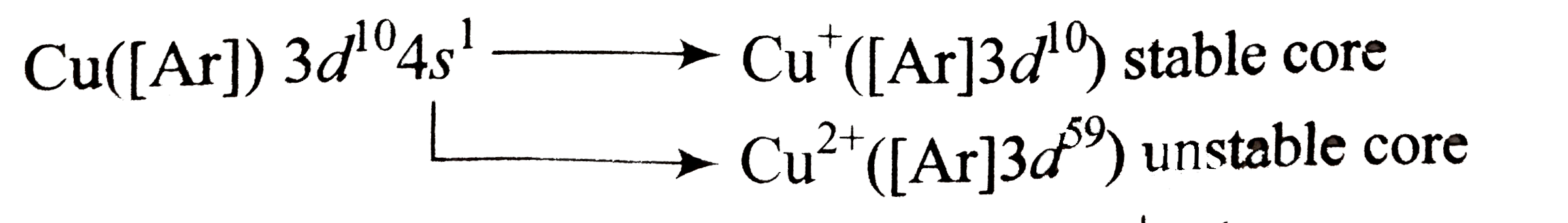A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 3|8 VideosCHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 4|15 VideosCHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 1|5 VideosAROMATIC HYDROCARBONS
R SHARMA|Exercise Archives|59 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS
R SHARMA|Exercise ARCHIVES|37 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL BONDING-Follow-up Test 2
- Most of the monatomic ions of the representative elements are obtained...
Text Solution
|
- Group 13 elements show less tendency to form ionic compounds than do g...
Text Solution
|
- No compounds of representative elements are found with ions having cha...
Text Solution
|
- There is a tendency for the elements of groups 13 to 15 of higher peri...
Text Solution
|
- The elements fo groups 16 and 17 whose atoms have the largest negative...
Text Solution
|
- Although the electron gain enthalpy of N(2s^2 2p63) is positive, the N...
Text Solution
|
- Which of the following cations posses neither noble gas nor pseudo nob...
Text Solution
|
- Many ions, particulary anions, are polyatomic. The atoms in these ions...
Text Solution
|
- Cations are usually made from metals and anions are usually made from ...
Text Solution
|
- Most transition metals from several cations having configurations.
Text Solution
|
- In forming ions, the atoms of transition metals generally lose the ns ...
Text Solution
|
- Many transition elements also form 3+ ions by losing one (n-1)d electr...
Text Solution
|
- In case of transition metals, certain atoms can lose different numbers...
Text Solution
|
- Many ionic compounds in transition metal ions are colored because of t...
Text Solution
|
- Aqueous solutions of transition metal cations are also colored. Which ...
Text Solution
|
- Which of the following catios forms colorless solution?
Text Solution
|
- Which of the following transition metal catios have noble gas core? ...
Text Solution
|
- For the formation of ionic bond between two atoms, the electronegativi...
Text Solution
|
- Although for Mg, DeltaiH(378 kJ mol^(-1)) is greater than DeltaiH2 (14...
Text Solution
|
- Although Na^(2+) has a higher charge and, therefore, NaCl2 should have...
Text Solution
|