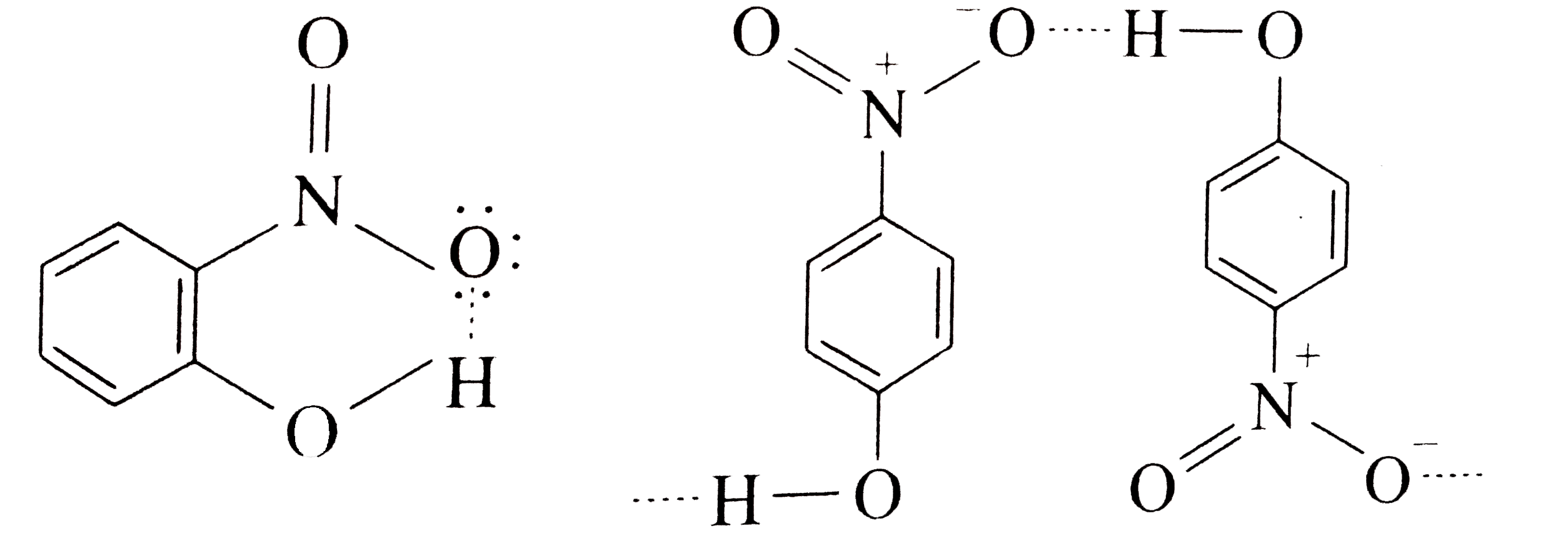
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 13|1 VideosCHEMICAL BONDING
R SHARMA|Exercise Question Bank (Level-I)|6 VideosCHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 11|8 VideosAROMATIC HYDROCARBONS
R SHARMA|Exercise Archives|59 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS
R SHARMA|Exercise ARCHIVES|37 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL BONDING-Follow-up Test 12
- Which of the following linear combinations of atomic orbitals is incor...
Text Solution
|
- The strongest hydrogen bonding exists in
Text Solution
|
- Which of the following has the highest boiling point?
Text Solution
|
- Coordination number of hydrogen in a hydrogen bond is
Text Solution
|
- The length of H bonds is
Text Solution
|
- Two ice cubes are pressed over each other until they unite to form one...
Text Solution
|
- The vapor pressure of o-nitrophenol at any given temperature is predic...
Text Solution
|
- Which of the following hydeides has the lowest boiling point?
Text Solution
|