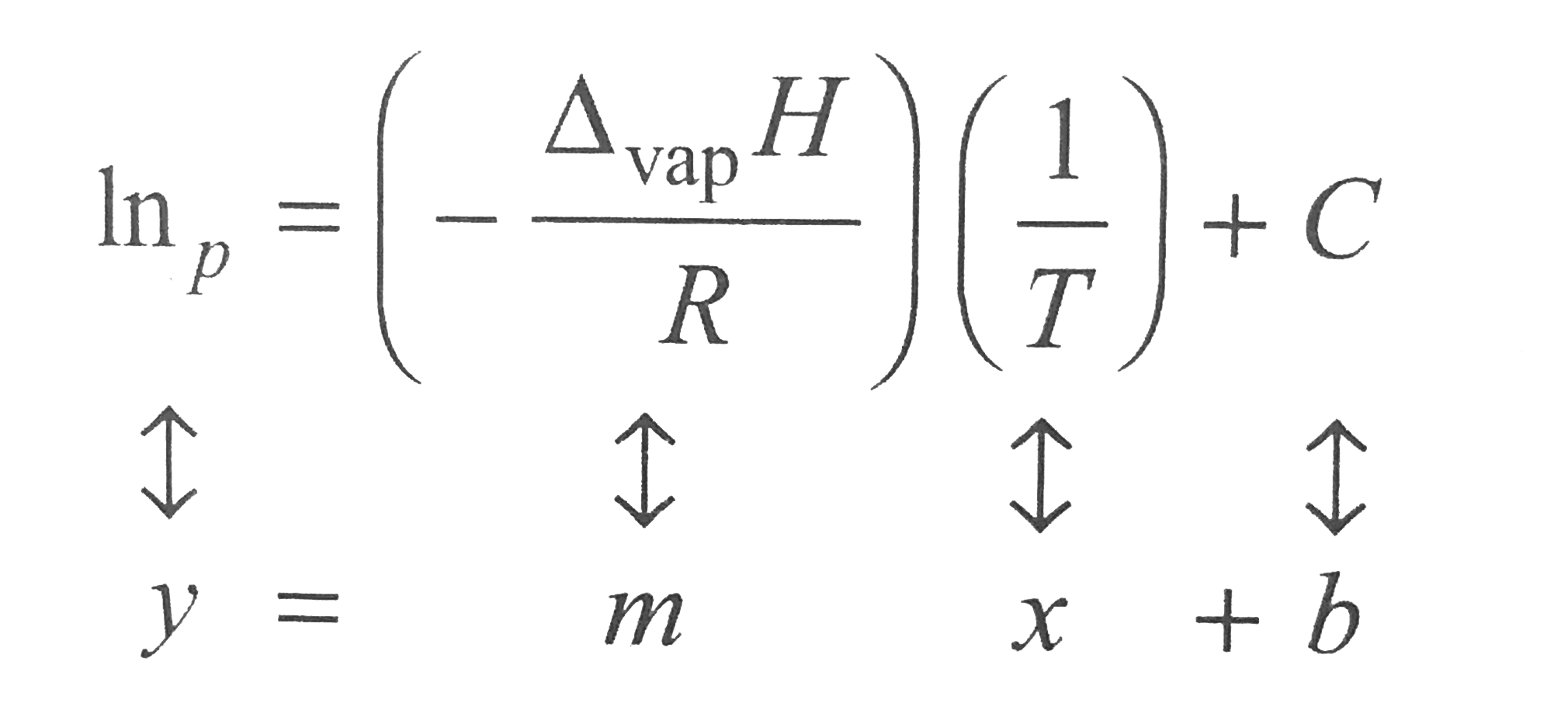A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
R SHARMA|Exercise Follow -up Test 11|8 VideosSTATES OF MATTER
R SHARMA|Exercise Follow -up Test 12|8 VideosSTATES OF MATTER
R SHARMA|Exercise Follow -up Test 9|8 VideosSOME BASIC CONCEPTS OF CHEMISTRY
R SHARMA|Exercise Archives|26 VideosSTRUCTURE OF ATOM
R SHARMA|Exercise ARCHIVES|55 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-STATES OF MATTER-Follow -up Test 10
- Which of the following is not true for liquids ?
Text Solution
|
- Which of the following is correct regarding the liquid state? (i) A ...
Text Solution
|
- At any given temperature , a certain number of molecules in a liquid p...
Text Solution
|
- The rate of evaporation ………. As temperature increases.
Text Solution
|
- Evaporation results in a ………… temperature in the liquid.
Text Solution
|
- As the concentration of molecules in the vapor phase increases, some m...
Text Solution
|
- Which of the following statements is correct anout a volatile liquid a...
Text Solution
|
- The partial pressure of vapor molecules above the surface of a liquid ...
Text Solution
|
- Vapor pressure of a liquid changes with …… of the liquid.
Text Solution
|
- Which of the following is the most volatile liquid?
Text Solution
|
- The boiling point of a liquid is the temperature at which the vapor pr...
Text Solution
|
- Which of the following is correct regarding boiling and evaporation ?...
Text Solution
|
- At the boiling point , bubbles form within the liquid because
Text Solution
|
- The quantitative relationship between the vapor pressure p of a liqui...
Text Solution
|
- At the critical temperature , the substance exists as a
Text Solution
|