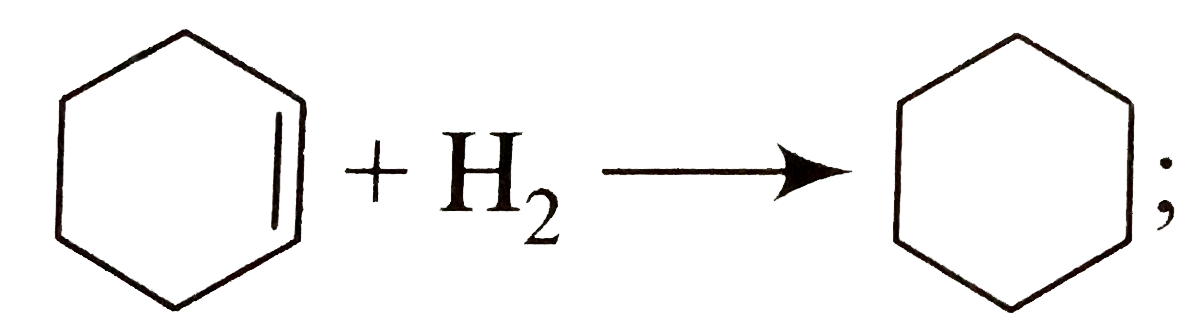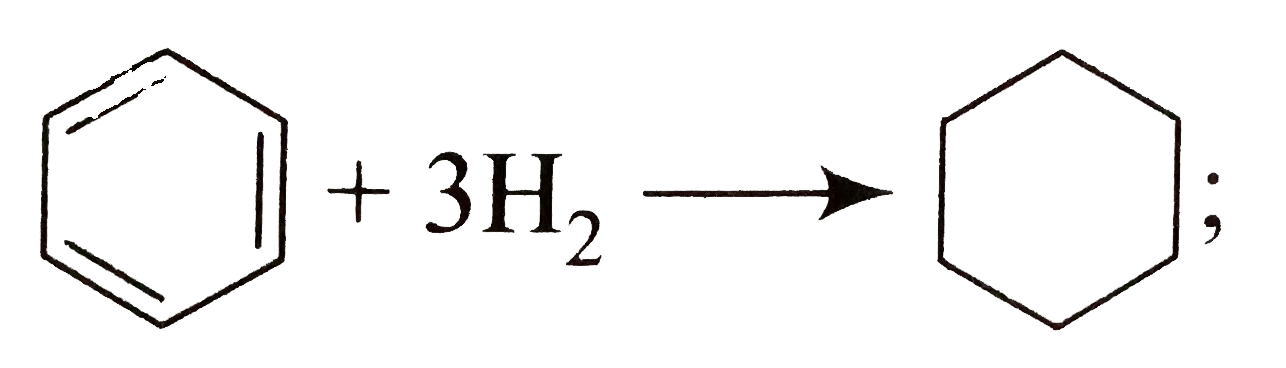A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-THERMODYNAMICS-Archives
- Calculate the enthalpy of formation of Delta(f)H for C(2)H(5)OH from t...
Text Solution
|
- The enthalpy change (Delta H) for the reaction N(2) (g) + 3 H(2) (g)...
Text Solution
|
- The enthalpy of hydrogenation of cyclohexene is -119.5kJ mol^(-1). If ...
Text Solution
|
- The enthalpy and entropy change for the reaction, Br(2)(l)+Cl(2)(g)r...
Text Solution
|
- Five mole of a gas put through a series of change as shown below graph...
Text Solution
|
- The heats fo neutralization of HCl with NH(4) OH and NaOH with CH(3) C...
Text Solution
|
- If the heat of neutralization for a strong acid - base reaction is -5...
Text Solution
|
- Which of the following is not correct?
Text Solution
|
- The absolute enthalpy of neutralization of the reaction, MgO(s)+2HCl...
Text Solution
|
- A reaction occurs spontaneously if:
Text Solution
|
- The standard molar heats of formation of ethane, carbon dioxide, and l...
Text Solution
|
- For the reaction of one mole of zinc dust with one mole of sulphuric a...
Text Solution
|
- The value of Delta H and Delta S for five different reaction are given...
Text Solution
|
- A cylinder of gas supplied by Bharat Petroleum is assumed to contain 1...
Text Solution
|
- One mole of a perfect gas expands isothermally to ten times its origin...
Text Solution
|
- For the reaction N(2) + 3 H(2) = 2 NH(3),
Text Solution
|
- In a reversible process, Delta S(sys) + Delta S(surr) is
Text Solution
|
- 1 mol of H(2) SO(4) in mixed with 2 mol of NaIH. The heat evolved will...
Text Solution
|
- It the enthalpy of vaporization of benzene is 308/ kJ mol^(-1) at boil...
Text Solution
|
- Internal energy is
Text Solution
|