Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-REDOX REACTIONS-Archives
- Balance the following equation is alkaline medium Zn(s) = N(3)^(-) ...
Text Solution
|
- Oxidation numbers of P in PO4^(3-), of S in SO4^(2-), and that of Cr...
Text Solution
|
- What is the stoichiometric coefficient fo Ca in the reaction ? Ca+Al...
Text Solution
|
- The number of moles of KMnO(4) that will be needed to react with one m...
Text Solution
|
- In the balanced chemical reaction IO(3)^(ө)+al^(ө)+bH^(ө)rarrcH(2)O+...
Text Solution
|
- The number of moles of KMnO(4) reduced by 1 "mol of" KI in alkaline me...
Text Solution
|
- Cr(2)O(7)^(2-)+Xoverset(H^(o+))rarrCr^(3+)+H(2)O+ "oxidised product" o...
Text Solution
|
- Which is the best description of the behaviour of bromine in the react...
Text Solution
|
- For the decolorization of 1 mol of KMnO4, the moles of H2O2 requiered...
Text Solution
|
- The elememt which forms oxides in all oxidation states +I to +V is.
Text Solution
|
- The oxidation number of carbon in . CH2Cl2 is .
Text Solution
|
- What is the net charge on ferrous ion ?
Text Solution
|
- Which of the following is the strongest oxidizing agent ?
Text Solution
|
- Which of the following is the most powerful reducing agent ?
Text Solution
|
- KI and CuSO4 solution when mixed give .
Text Solution
|
- What is the equivalent mass of IO4^(-) when it is converted into I2 i...
Text Solution
|
- In acidic medium, dichromate ion oxidizes ferrous ion to ferric ion. I...
Text Solution
|
- Which of the following is both oxidizing as well as reducing agent ?
Text Solution
|
- P(4)+NaOH+H(2)O rarr NaH(2)PO(3)+PH(3) is
Text Solution
|
- MnO(4)^(2-) (1 mole) in neutral aqueous medium is disproportionate to
Text Solution
|
- The oxidation states of sulphur in the anions SO(3)^(2-), S(2)O(4)^(2-...
Text Solution
|
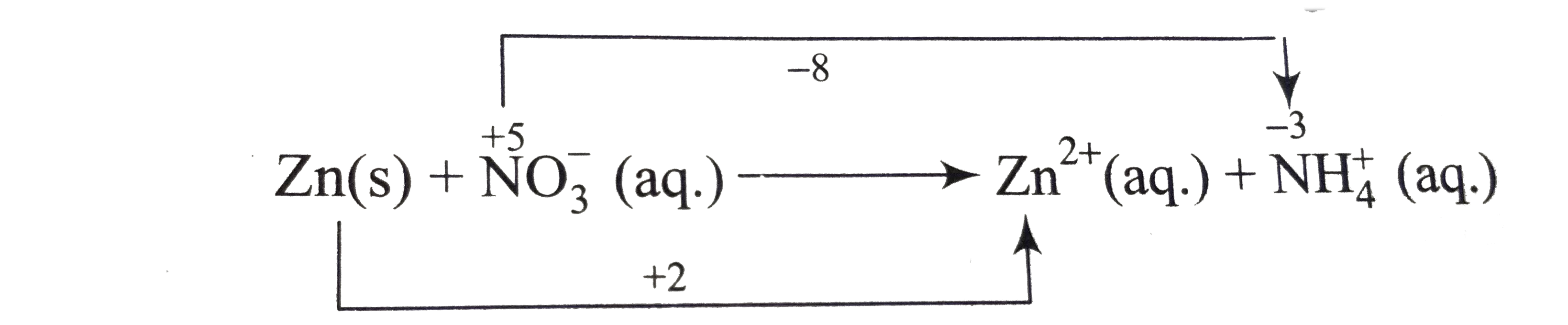 .
.