A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
R SHARMA|Exercise Follow-up Test 6|10 VideosREDOX REACTIONS
R SHARMA|Exercise Follow-up Test 7|10 VideosREDOX REACTIONS
R SHARMA|Exercise Follow-up Test 4|15 VideosPURIFICATION AND CHARATERIZATION OF ORGANIC COMPOUNDS
R SHARMA|Exercise Archives|38 VideosSOME BASIC CONCEPTS OF CHEMISTRY
R SHARMA|Exercise Archives|26 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-REDOX REACTIONS-Follow-up Test 5
- In the reaction x Mg + yHNO3 rarr Mg (NO(3))(2) + N2O+ H2 O.
Text Solution
|
- In the reaction x FeS2 + yO2 rarr Fe2 O3 + SO2
Text Solution
|
- In the reaction xAs2S3 + y NO3^(-)+ H^(+) rarr AsO4^(3-) + S + NO +...
Text Solution
|
- In the reaction xCl2 + yOH^(-) rarr Cl^(-) + ClO3^(-) .
Text Solution
|
- The value of n in NO3^(-) + 4H^(+) + n e^(-) rarr 2 H2O + NO is .
Text Solution
|
- The number of electrons transferred (lost and gained ) during the reac...
Text Solution
|
- Consider the follwing reaction in basic medium : NH3 + O Cl^(-) rarr...
Text Solution
|
- In the following reaction, the values fo x,y and z respectively, are ...
Text Solution
|
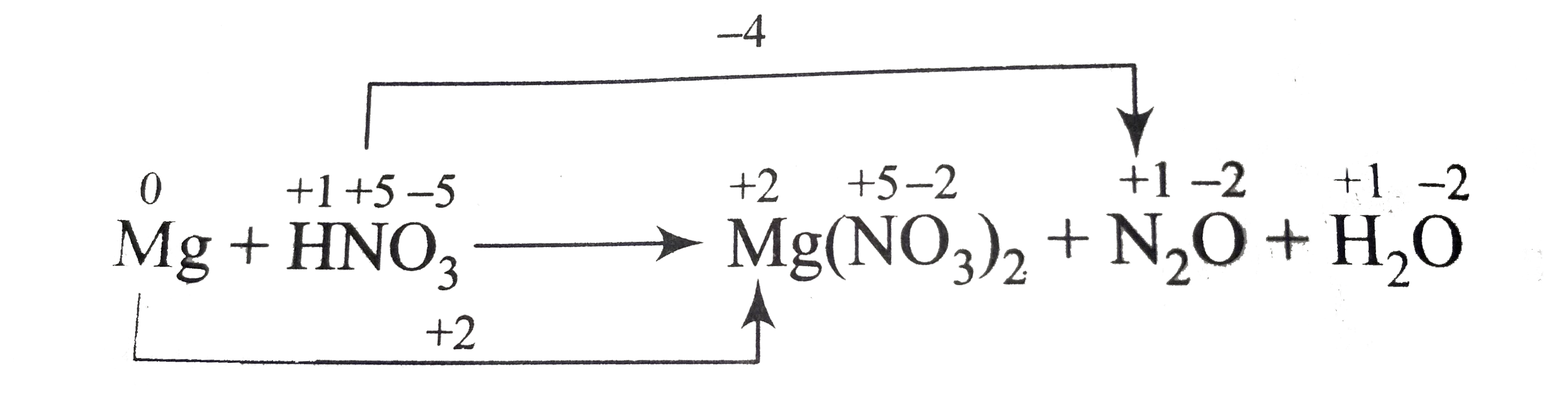 .
.