A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-REDOX REACTIONS-Question Bank
- The reaction P4 + 3 NaOH + 3 H2 O rarr 3 NaH2 PO + PH3 is an exampl...
Text Solution
|
- Which of the following is a set fo reducing agents ?
Text Solution
|
- Which fo the following is a redox reaction ?
Text Solution
|
- Compound CrO5 has structure as shown ltbtgt The oxidation number f...
Text Solution
|
- Which of the following chemical reactions depects the oxidizing behave...
Text Solution
|
- Which fo the following will not be oxidized by O3 ?
Text Solution
|
- aK(2)Cr(2)O(7)+bKCl+cH(2)SO(4)rarrxCrO(2)Cl(2)+yKHSO(4)+zH(2)O The a...
Text Solution
|
- The pair fo compounds having metals in their highest oxidation state i...
Text Solution
|
- which of the following is a redox reaction ?
Text Solution
|
- Oxidation numbers fo iodine in IO3^(-), IO4^(-), Kl, and I3 , respect...
Text Solution
|
- In which fo the following has the oxidation number of oxygen been arra...
Text Solution
|
- Consider a titration of potassium dichromate solution with acidified M...
Text Solution
|
- Potassium iodide reacts with acidified K(2)Cr(2)O(7). How many moles o...
Text Solution
|
- Excess of KI reacts with CuSO(4) solution and Na(2)SO(3) solution is a...
Text Solution
|
- When KMnO(4) acts as an oxidising agnet and ultimetely from MnO(4)^(2-...
Text Solution
|
- When Kl is added to acidified solution fo sodium nitrite,
Text Solution
|
- The oxidation number of Cl in CaOCl(2) is
Text Solution
|
- Which fo the following statements are correct concerning redox propret...
Text Solution
|
- What products are expected from the desproprtionation reactin of hypoc...
Text Solution
|
- The oxidation number of S in H(2)S(2)O(8) is
Text Solution
|
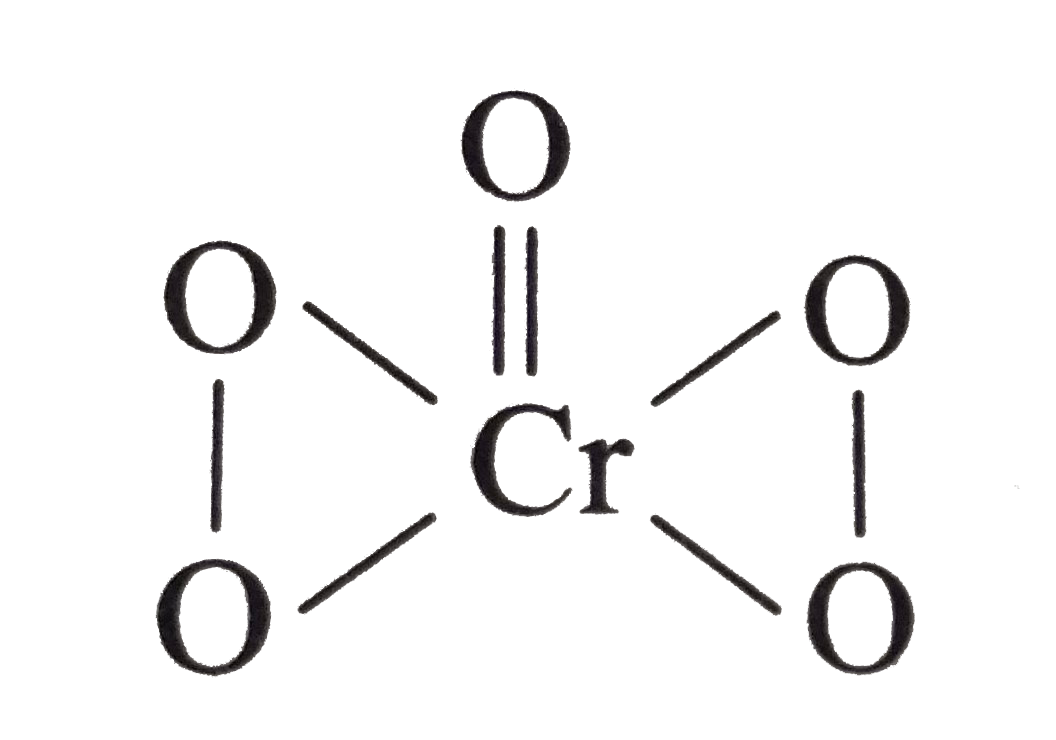 ltbtgt The oxidation number fo Cr in the above compound is .
ltbtgt The oxidation number fo Cr in the above compound is .