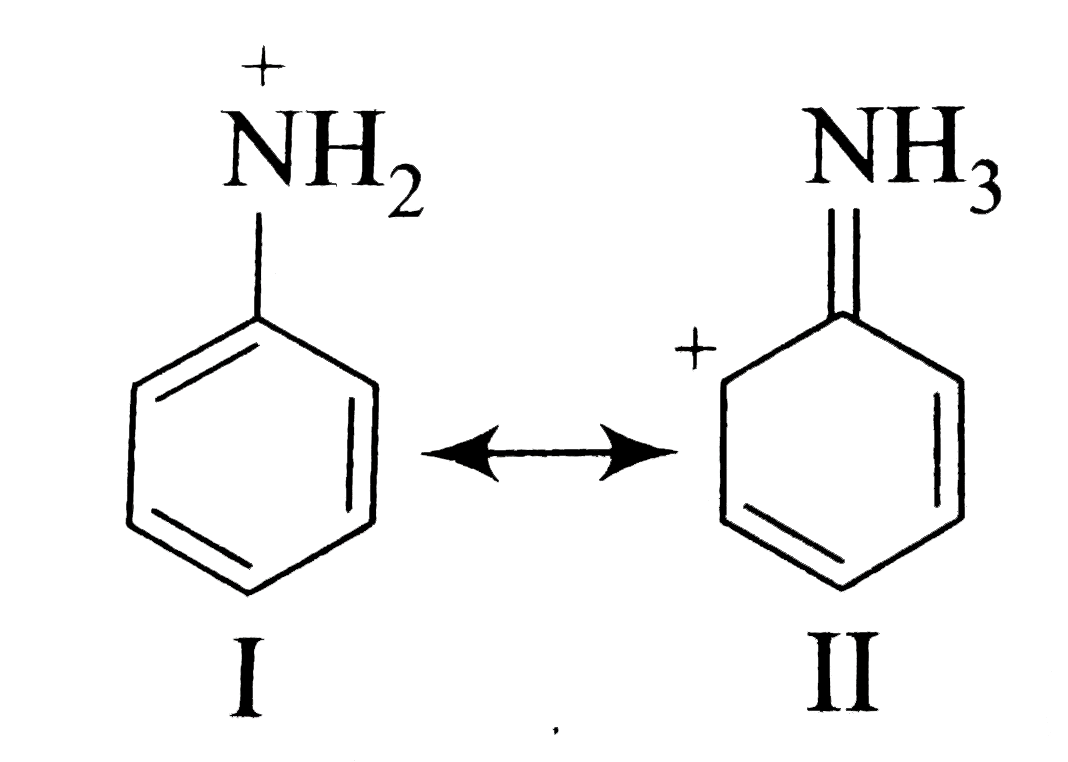
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
R SHARMA|Exercise Follow- up Test 8|9 VideosGENERAL ORGANIC CHEMISTRY
R SHARMA|Exercise Follow- up Test 9|6 VideosGENERAL ORGANIC CHEMISTRY
R SHARMA|Exercise Follow- up Test 6|10 VideosEQUILIBRIUM
R SHARMA|Exercise ARCHIVES|69 VideosISOMERISM
R SHARMA|Exercise Archives|11 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-GENERAL ORGANIC CHEMISTRY-Follow- up Test 7
- Which of the following groups shows the strongest -R effect?
Text Solution
|
- Which of the following has +R (or +M) effect?
Text Solution
|
- Which of the following groups shows the weakest +R effect?
Text Solution
|
- In which of the following compounds, does the substituent not exert it...
Text Solution
|
- Examine the following two structures for the anilinium ion and choose ...
Text Solution
|
- Resonance structure of a molecule cannot have
Text Solution
|
- Which of the following resonating structures of 1-methoxy-1,3-butadien...
Text Solution
|
- The most unlikely representation of resonance structure of p-nitrophen...
Text Solution
|
- Unlike other amines (RNH(2)), guanidine, H(2)overset(..)N-underset(..)...
Text Solution
|
- Phenols are more acidic than alcohols because
Text Solution
|
- Although phenoxide ion has more number of resonating structures than c...
Text Solution
|