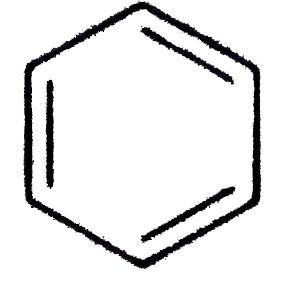
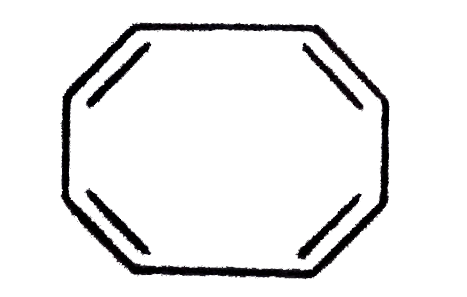
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALKYNES-Follow-up Test 3
- Acetylene on reaction with 1 mol of HCl in the presence of mercuric ch...
Text Solution
|
- Which of the following is the least acidic?
Text Solution
|
- Lewisite, a chemical weapon, is prepared by the reaction of acetylene ...
Text Solution
|
- Acetylene polymerizes in the presence of Ni(CN)2 under pressure to for...
Text Solution
|
- Acetylene underset(NH4Cl)overset(CuCl)to The product formed in the rea...
Text Solution
|
- Acetylene is transported from one place to another in the form of tis ...
Text Solution
|
- When acetylene reacts with an excess of hypochlorous acid, the product...
Text Solution
|
- When acetylene is passed inito warm acetic acid (excess) in the presen...
Text Solution
|
- HC-=CH+CH3OHunderset(150-160^@)overset(CH3OK)toP Identify the produc...
Text Solution
|
- HC-=Chunderset(u n excess)overset(NaNH2)toIunderset(2. H2O)overset(1. ...
Text Solution
|
- Which of the following compounds on heating with zinc powder in alcoho...
Text Solution
|
- Which of the reaction is not feasible?
Text Solution
|