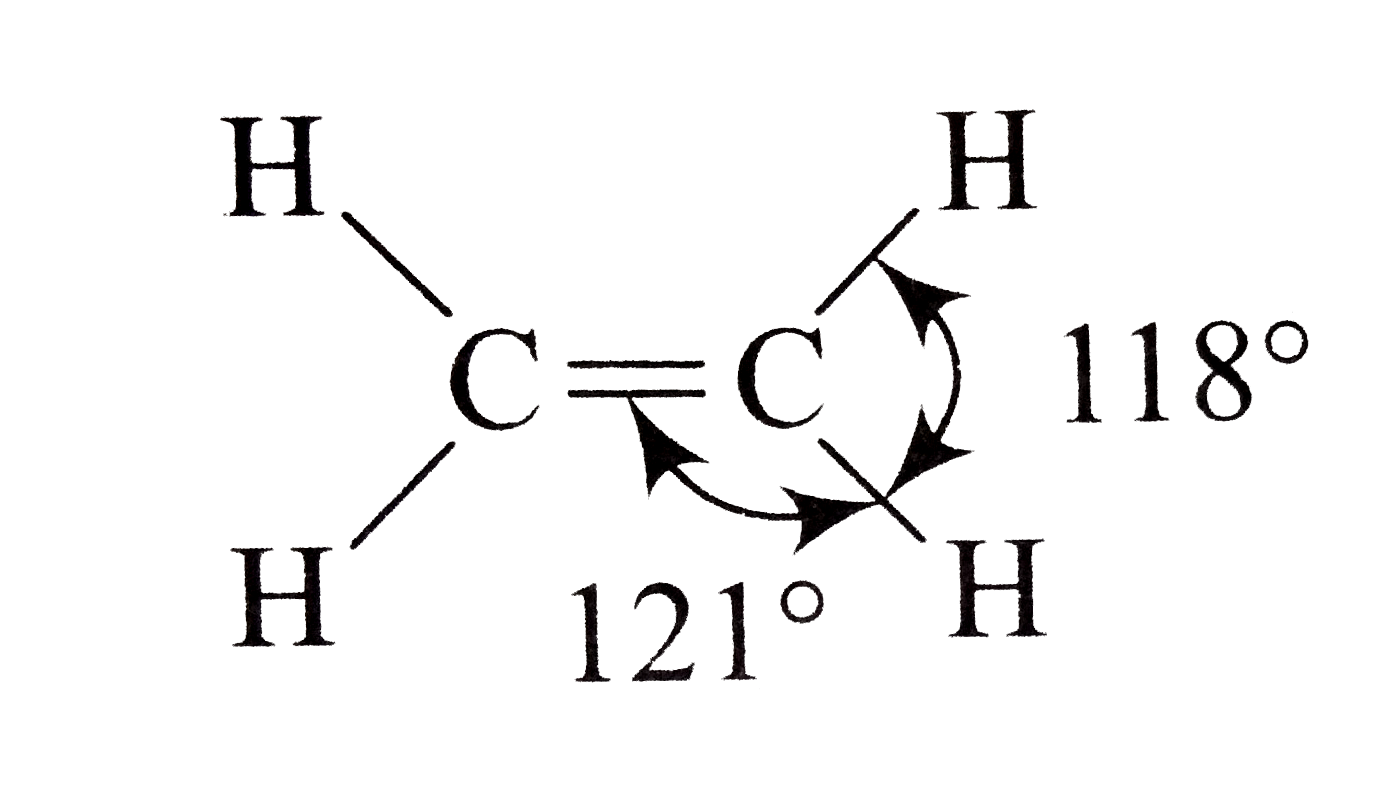
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALKENES-Follow-up-Test 1
- Alkenes are unsaturated acyclic hydrocarbons whose molecules contain …...
Text Solution
|
- Which of the follwing alkenes was called olefiant gas?
Text Solution
|
- The C-C distance in ethane is
Text Solution
|
- The H-C=C ( or C=C-H) bond angle in ethane is
Text Solution
|
- The maximum number of atoms that might exist in one plane in "but-2-en...
Text Solution
|
- Which of the following statements is incorrect ?
Text Solution
|
- In Coverset(a)-C=Coverset(b)-C=Coverset(c )-Coverset(d)-C the stro...
Text Solution
|
- What is the degree (or element) of unsaturation for C(4)H(8)?
Text Solution
|
- The possible number of isomers having the formula C(3)H(6) is
Text Solution
|
- The IUPAC name of the following alkene is
Text Solution
|
- The IUPAC name of the allyl group is
Text Solution
|
- Which of the follwing exhibits geometric isomerism?
Text Solution
|
- How many alkenyl group can be derived from propane?
Text Solution
|
- The interconversion of cis-and trnas-isomers of an alkene is possible ...
Text Solution
|