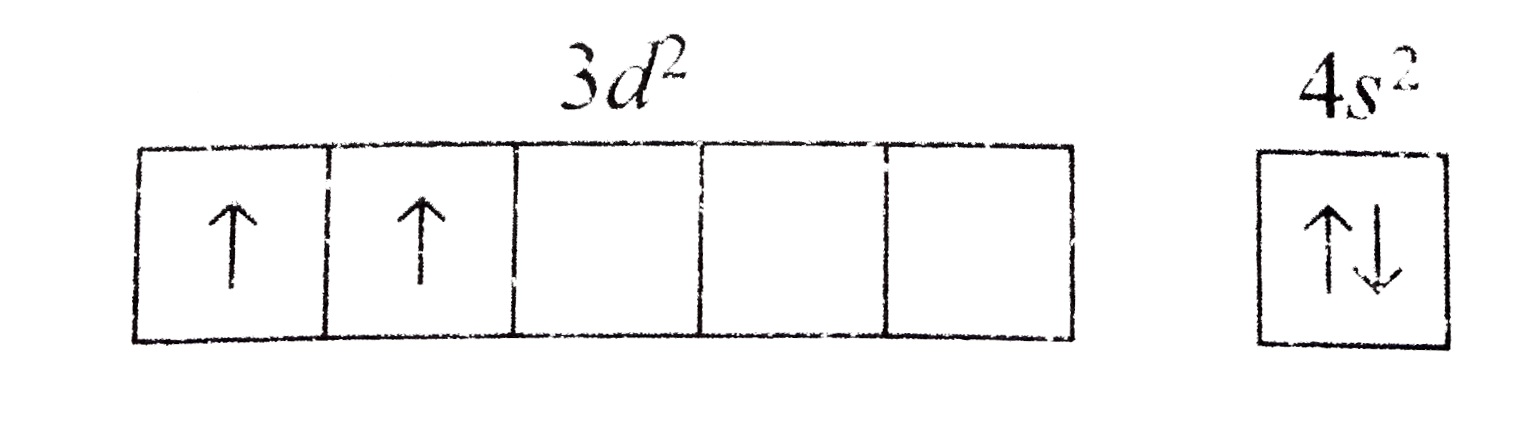A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE D AND F BLOCK ELEMENTS
R SHARMA|Exercise Follow-up Test 4|18 VideosTHE D AND F BLOCK ELEMENTS
R SHARMA|Exercise Follow-up Test 5|9 VideosTHE D AND F BLOCK ELEMENTS
R SHARMA|Exercise Follow-up Test 2|21 VideosSURFACE CHEMISTRY
R SHARMA|Exercise Archives|18 VideosTHE P BLOCK ELEMENTS
R SHARMA|Exercise Archieves|62 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-THE D AND F BLOCK ELEMENTS-Follow-up Test 3
- Which of the following statements is context of paramagnetic materils ...
Text Solution
|
- Which of the following is not ferromagnetic ?
Text Solution
|
- Which of the following statements is incorrect ?
Text Solution
|
- Calculate the magnetic moment of a divalent ion in aqueous solution if...
Text Solution
|
- The 'spin only' magnetic moment of Ti in a given compound is 1.73 BM. ...
Text Solution
|
- Which of the following metal ions is colored in aqueous solution ?
Text Solution
|
- Which of the following is coloured ?
Text Solution
|
- The transition metals have a strong tendency to form complaexes becaus...
Text Solution
|
- Which of the following is arranged in order of increasing stability of...
Text Solution
|
- The interstitial compounds of transition metals are than the metal its...
Text Solution
|
- Which of the following is used in large amount to make ferrous and non...
Text Solution
|