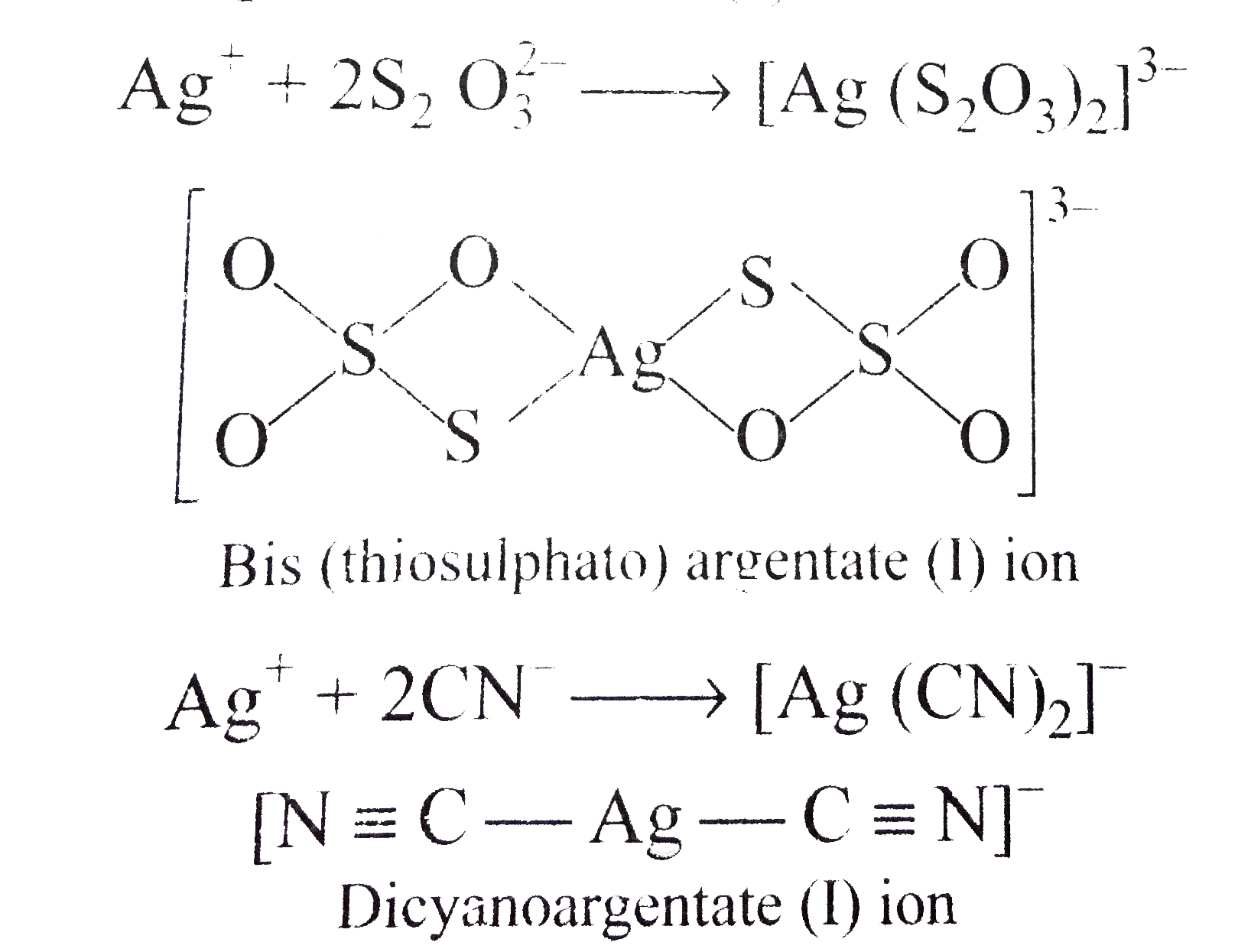A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-THE D AND F BLOCK ELEMENTS-Question Bank (Building the Knowledge)
- Which of the following is incorrect ?
Text Solution
|
- Silver halides darken in light owing to ------- , a property which is ...
Text Solution
|
- The precipitate of AgCI dissolves in an excess of NH(3) , KCN and Na(2...
Text Solution
|
- When a solution of mercuric chloride treated with an excess of stannic...
Text Solution
|
- Which of the following is not correctly matched ?
Text Solution
|
- The most common oxosalt of copper (II) is blue virtiol. It has the com...
Text Solution
|
- How many water molecules in blue vitriol are coordinated to the metal ...
Text Solution
|
- If a aqueous solution of copper (II) sulphate is saturated with ammoin...
Text Solution
|
- Which of the following has more number of unpaired d-electrons ?
Text Solution
|
- K(2)Cr(2)O(7) on heating with aqueous NaOH gives
Text Solution
|
- Cuprous compounds such as CuCI, CuCN and CuSCN are the only salts stab...
Text Solution
|
- Stainaless steel contains iron and
Text Solution
|
- Actinides
Text Solution
|
- When (NH(4))(2)Cr(2)O(7) is heated, the gas evolved is
Text Solution
|
- The common oxidation states of Ti are
Text Solution
|
- When CuSO(4) is electrolysed, using Pt electrodes
Text Solution
|
- The general valence shell electronic configuration of transition eleme...
Text Solution
|
- While extracting an element from its ore, the ore is grind and leached...
Text Solution
|
- Within each transition series, the oxidation states
Text Solution
|
- In which of the following compounds transition metal has zero oxidatio...
Text Solution
|