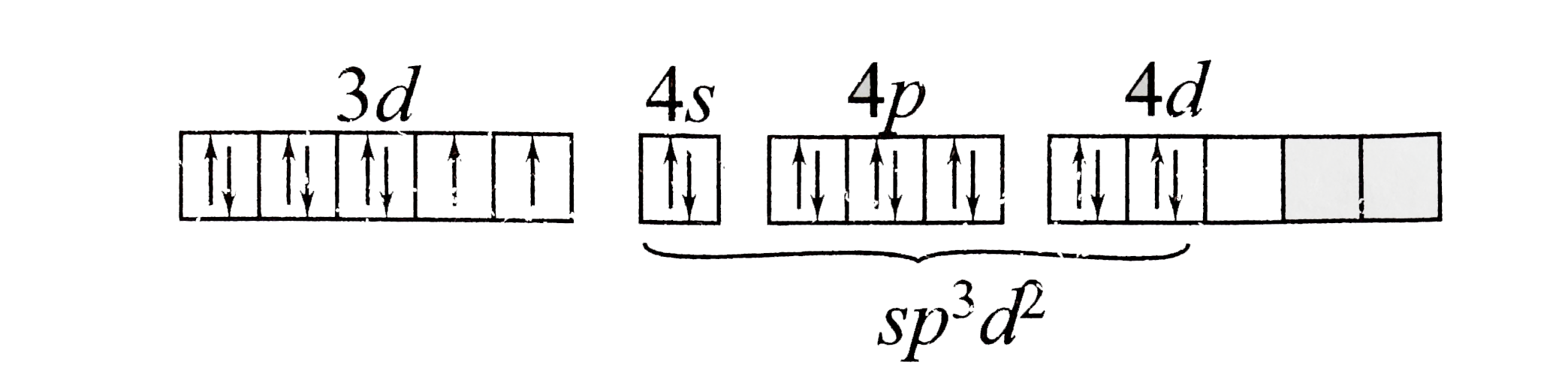
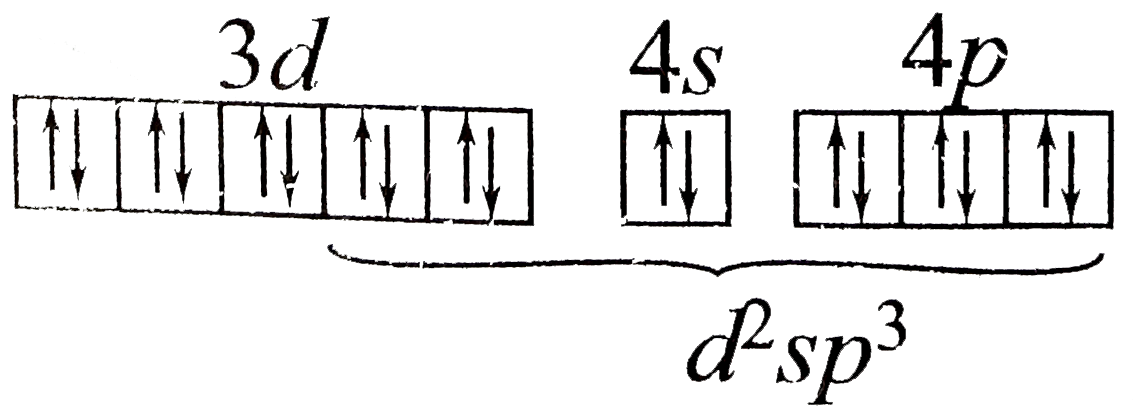
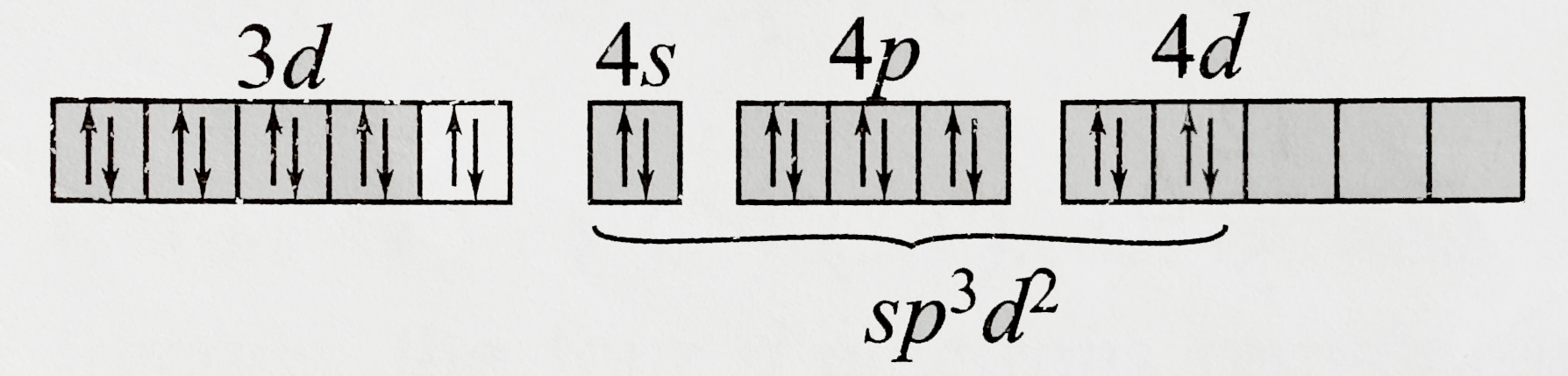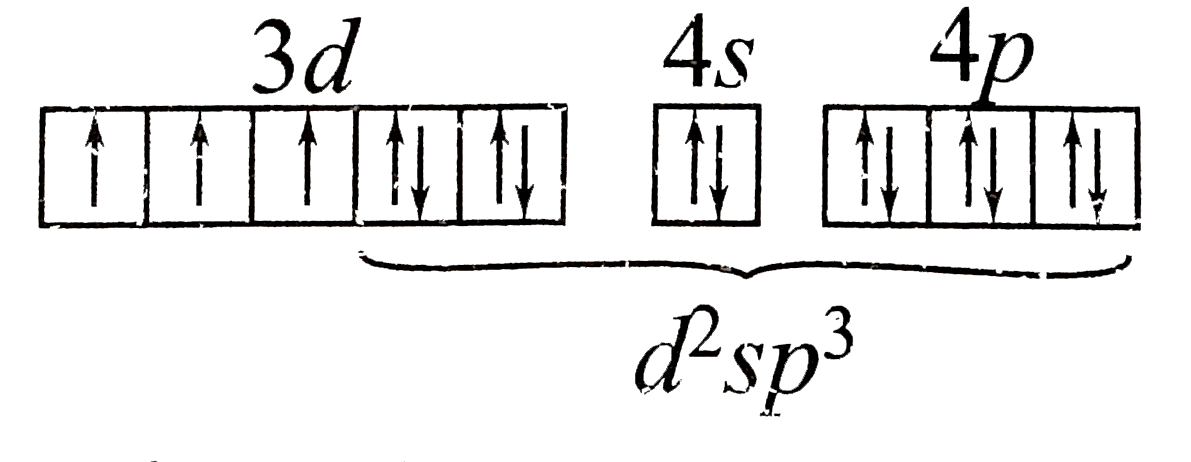A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-COORDINATION COMPOUNDS-Archives
- A magnetic moment of 1.73 B.M. will be shown by one among the followin...
Text Solution
|
- An excess of AgNO(3) is added to 100mL of a 0.01M solution of dichloro...
Text Solution
|
- Which one of the following is an outer orbital complex and exhibits pa...
Text Solution
|
- Red precipitae is obtained when ethanol solution of dimethylglyoxime i...
Text Solution
|
- Low spin complex of d^6-cation in an octahedral field will have the fo...
Text Solution
|
- The complex, [Pt(py)(NH3)BrCl] will have how many geometrical isomers?
Text Solution
|
- [Co(HN(3))(6)][Cr(CN)(6)] and [Cr(NH(3))(6)][Co(CN)(6)] are .
Text Solution
|
- The d-electron configurations of Cr^(2+), Mn^(2+), Fe^(2+) and Co^(2+)...
Text Solution
|
- Of the following complex ions, which is diamagnetic in natures?
Text Solution
|
- Which of the following complex compounds will exhibit highest magnetic...
Text Solution
|
- Which of the following complex ions is not expected to absorve visible...
Text Solution
|
- The existence of two different colored complexes with the composition ...
Text Solution
|
- Crystal field stabilization energy for high spin d^4 octahedral comple...
Text Solution
|
- Which one of the following complex is not expected to exhibit isomeris...
Text Solution
|
- Which of the following does not show optical isomerism ?
Text Solution
|
- Which of the following complex compounds will exhibit highest magnetic...
Text Solution
|
- A 0.002 M aqueous solution of an ionic compound [Co(NH(3))(5)(NO(2))] ...
Text Solution
|
- Which of the following complexes exhibits the highest paramagnetic beh...
Text Solution
|
- In which of the following coordination entites the magnitude of Delta(...
Text Solution
|
- The d electron congfiguration of Cr^(2+) , Mn^(2+) , Fe^(2+) and Ni^(2...
Text Solution
|