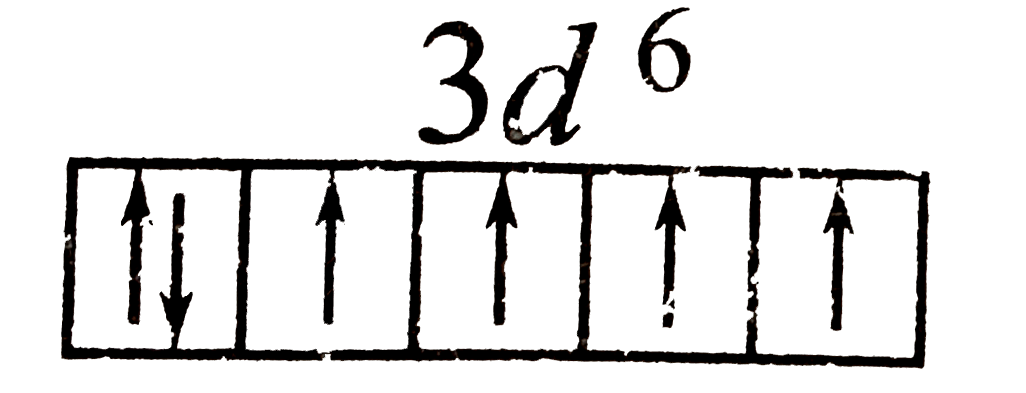A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-COORDINATION COMPOUNDS-Archives
- Considering H2O as a weak field ligand, the number of unpaired electro...
Text Solution
|
- CN^(-) is a strong field ligand. This is due to the fact that
Text Solution
|
- Which of the following does not have a metal carbon bond?
Text Solution
|
- Which of the following is considered to be an anticancer species ?
Text Solution
|
- Which of the following coordination compounds would exhibit optical is...
Text Solution
|
- Among the following, which is not the pi - bonded organometallic compo...
Text Solution
|
- The number of unpaired electrons in the complex ion [CoF6]^(3-) is (At...
Text Solution
|
- According to IUPAC nomenclature sodium nitroprusside is named as
Text Solution
|
- Which of the following octahedral complex does not show geometrical is...
Text Solution
|
- The hypothetical complex triamminediaquachloridocobalt(III) chloride c...
Text Solution
|
- Atomic numbers of Cr and Fe are respectively 24 and 26. Which of the f...
Text Solution
|
- In the silver plating of copper, K[Ag(CN)(2)] is used instead of AgNO(...
Text Solution
|
- Which of the following will give maximum number of isomer ?
Text Solution
|
- Which of the following organometallic compound is sigma and pi-bonded?
Text Solution
|
- Coordination number of Ni in [Ni(C2O4)3]^(4-) is:
Text Solution
|
- Which statement is incorrect?
Text Solution
|
- Which of the following will exhibit maximum ionic conductivity ?
Text Solution
|
- Which of the following complexes will have four isomers ?
Text Solution
|
- In the separation of Cu^(2+) and Cd^(2+) of Iind group in qualitative ...
Text Solution
|
- What is the shape of Fe(CO)(5) molecule ? Given that its dipole moment...
Text Solution
|