A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-STEREOCHEMISTRY-Follow-up Test
- Which of the following compounds possesses a stereocenter?
Text Solution
|
- The number of chiral centres present in 3,4-dichloropentan-2-ol is
Text Solution
|
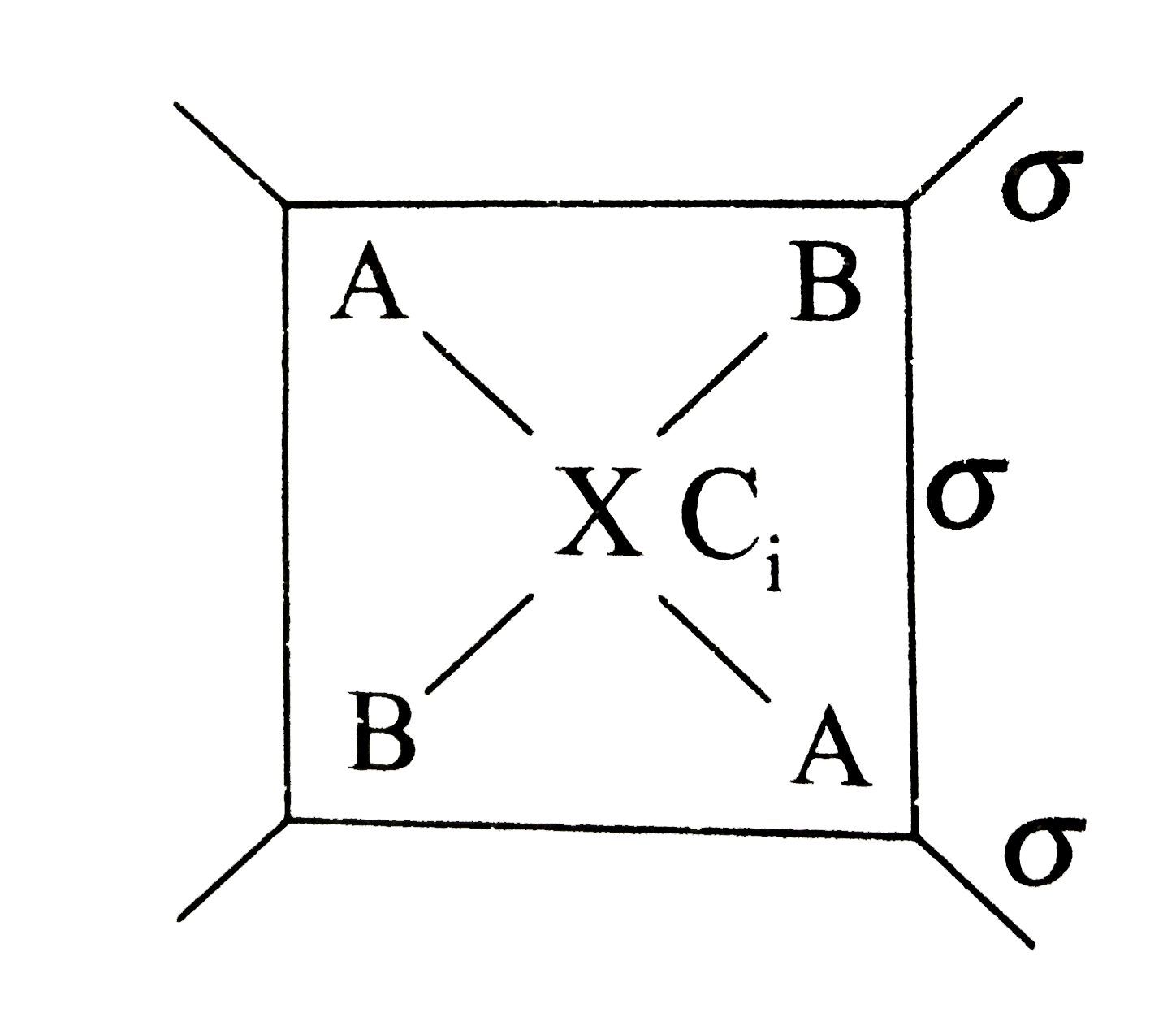
- Consider a hypothetical planar molecule with the formula A(2)B(2)X(whe...
Text Solution
|
- Which of the following can not be a chiral center?
Text Solution
|
- Which of the following pairs of compounds are enantiomers?
Text Solution
|
- Enantiomers have identical
Text Solution
|
- Which of the following statements in not correct?
Text Solution
|
- Enantiomerism is shown by
Text Solution
|
- Which of the following capital letters is achiral?
Text Solution
|
- Which of the following is a racemic mixture?
Text Solution
|
- The process of conversion of an optically pure enantiomer into an opti...
Text Solution
|
- The process of separating a racemic mixture into optically pure (+) an...
Text Solution
|
- Enantiomers can be separated by means of
Text Solution
|
- Dextrororatory bytan-2-ol has a specific rotation [alpha](D)^(25) =+13...
Text Solution
|
- Dextrororatory alpha-pinene has a specific rotation [alpha](D)^(20) =+...
Text Solution
|
- The specific rotation of a pure enantiomers is +12^(@). What will be i...
Text Solution
|
- According to CIP sequence rule, the correct arrangement in order of de...
Text Solution
|
- Which of the following structures has the R-configuration at the chir...
Text Solution
|
- Which of the following structures has the S-configuration at the chira...
Text Solution
|
- Which of the following structures has the D-configuration
Text Solution
|