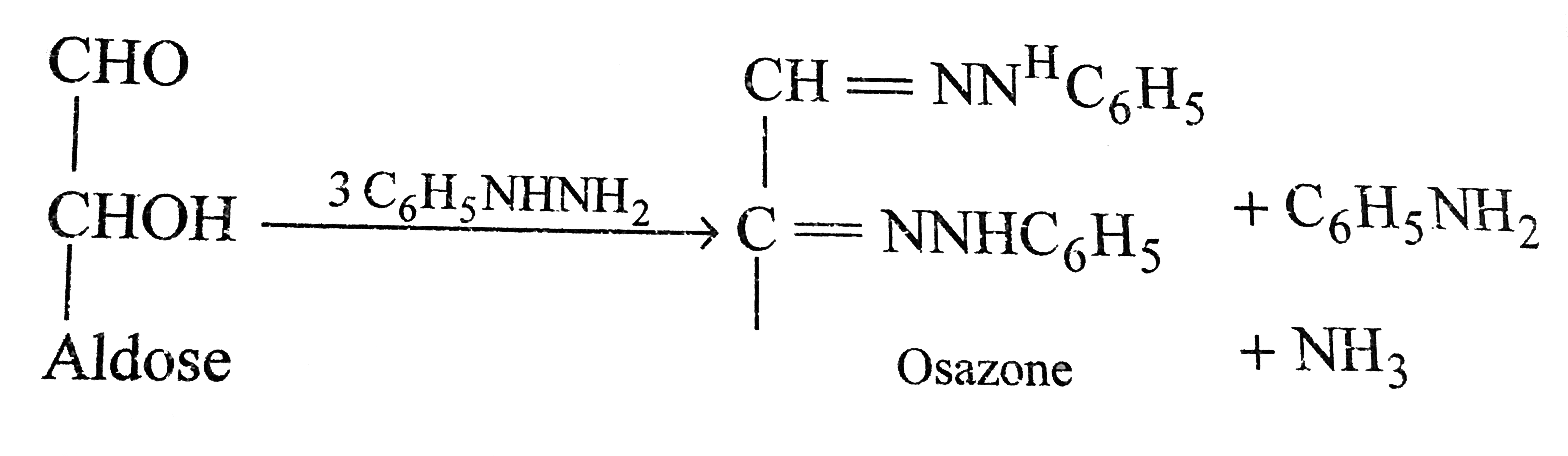
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-BIOMOLECULES-Question Bank Level - III
- Which of the following monosaccharides is a pentose?
Text Solution
|
- The number of stereoismers of aldepentose, excluding cyclic forms is
Text Solution
|
- Which of the following is known as laevulose?
Text Solution
|
- Which of the following is the open-chain structure of D-glucose?
Text Solution
|
- In an aqueous solutions of D-glucose all the following cyclic forms, b...
Text Solution
|
- Which of the following compounds does not undergo oxidative cleavage w...
Text Solution
|
- When glucose is treated with an execss of HIO(4), the products formed ...
Text Solution
|
- The final product of which of following reactions furnishes evidence t...
Text Solution
|
- D-Glucose and D-fructose both form the same osazone, Which statements ...
Text Solution
|
- From a mixture of amino acids the constituents can be separated by
Text Solution
|
- When gamma, delta- and epsilon- amino acids are heated of them are con...
Text Solution
|
- Proteins that contains a nonprotein group bonded to the protein part a...
Text Solution
|
- In E. coli DNA, AT//GC ratio is 0.93. If the number of moles of adeni...
Text Solution
|
- Glucose molecule reacts with X number of molecules of phenylhydrazine ...
Text Solution
|