A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-BIOMOLECULES-Question Bank Level - IV
- The IUPAC name of the ketotriose is
Text Solution
|
- Which of the following is D-xylose
Text Solution
|
- The number of ketotetroses with respect to constitution and configurat...
Text Solution
|
- In D-erythrose, the configurations at C - 2 and C - 3 respectively
Text Solution
|
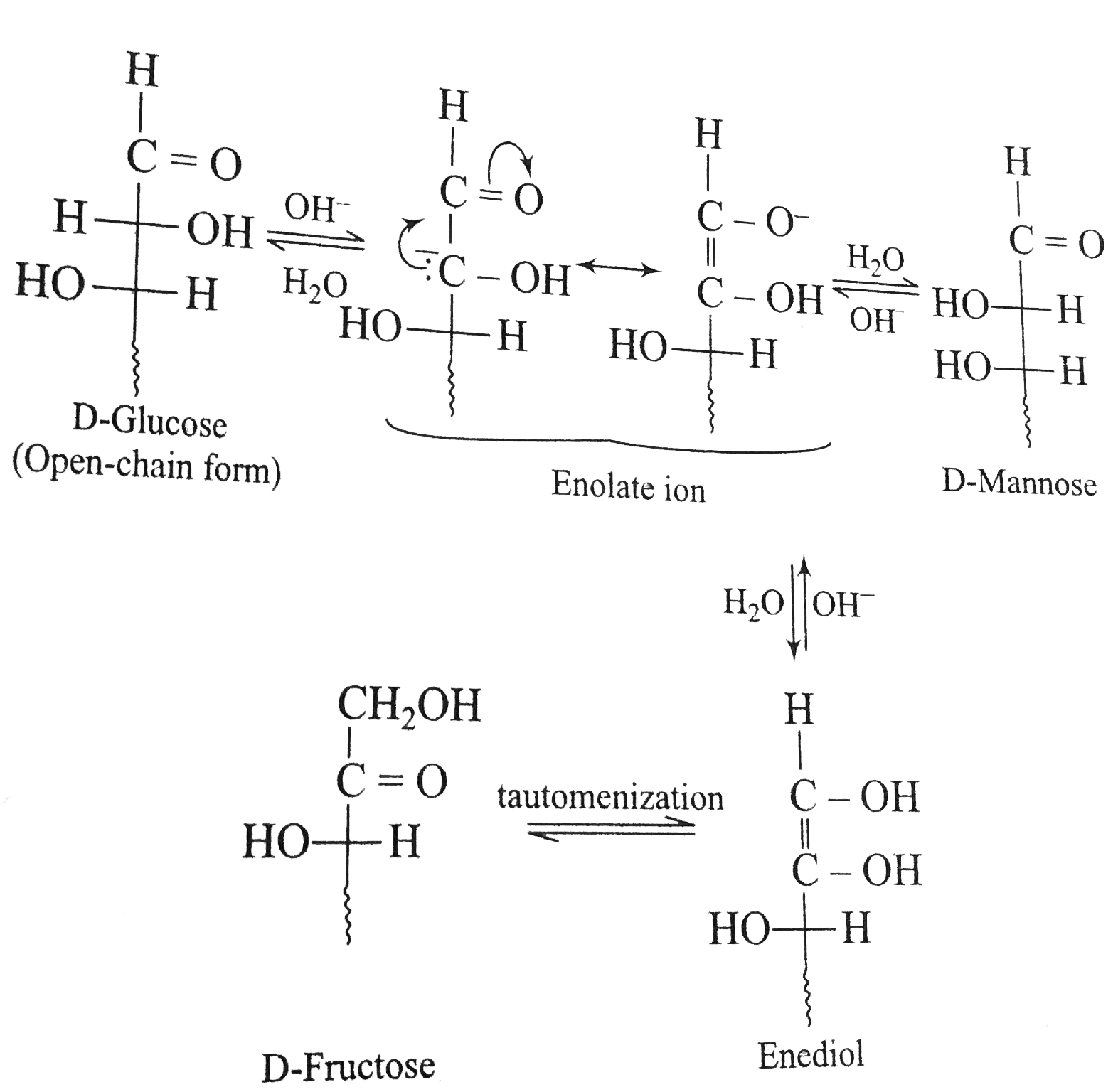
- Dissolving monosaccharaides in aqueous base causes them to undergo
Text Solution
|
- A methyl glucoside can be converted to the anhydride in the presence ...
Text Solution
|
- D-glucose is treated with an excess of acetic anhydride in the presenc...
Text Solution
|
- For the conversion of one molecule of glucose into one molecule of glu...
Text Solution
|
- Which of the following is used for making mucilage for postal stamps?
Text Solution
|
- The following species has three acidic sites, namely -COOH (denoted as...
Text Solution
|
- In sickle cell anaemia, the basis of malfunction of haemoglobin molecu...
Text Solution
|