Text Solution
Verified by Experts
CP SINGH-KINETIC THEORY OF GASES-Exercises
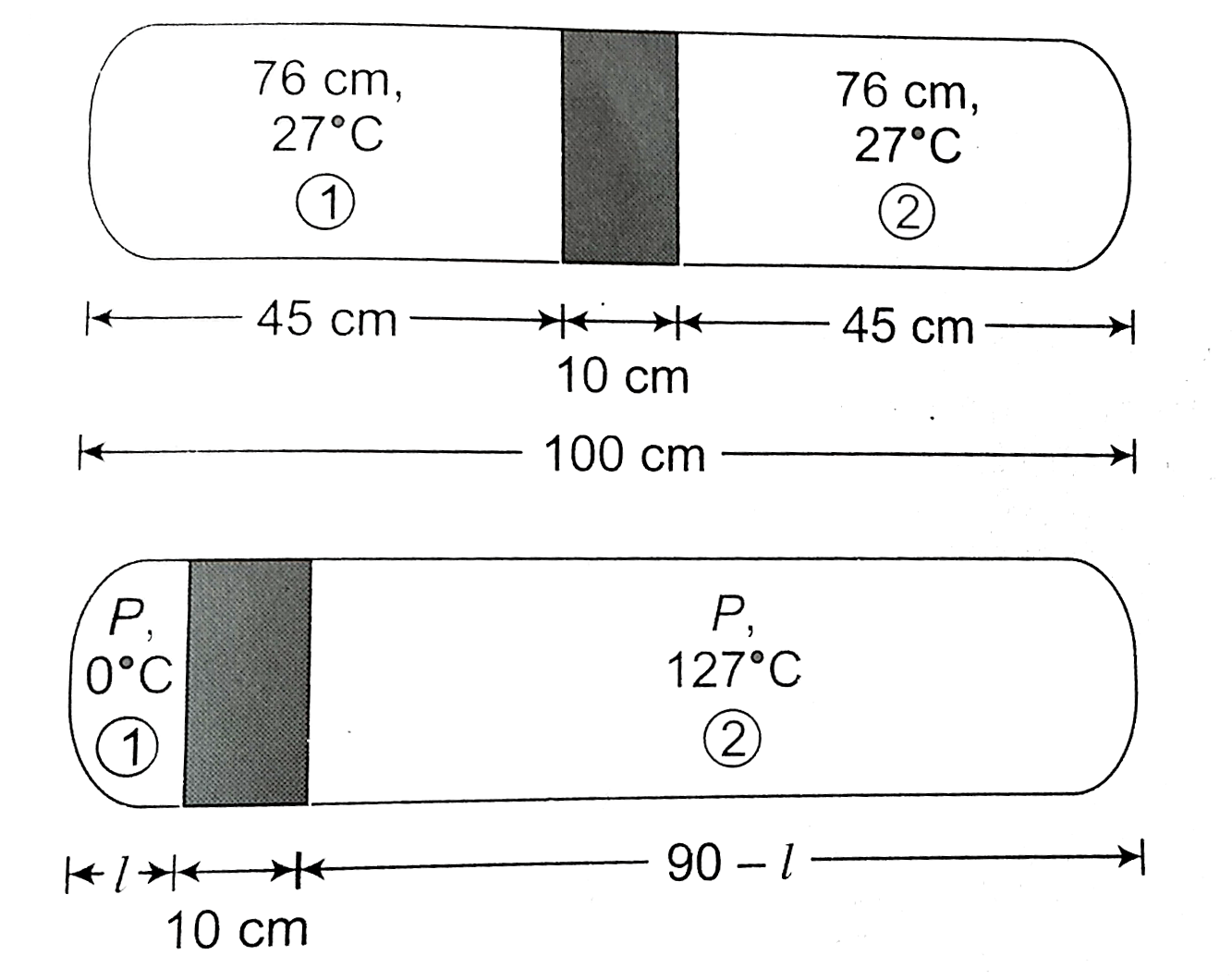
- A glass tube sealed at both ends is 100cm long, It lies horizontally w...
Text Solution
|
- A gas behaves more closely as an ideal gas at
Text Solution
|
- The mass of 1 litre of helium under a pressure of 2 atm and at a tempe...
Text Solution
|
- A gas is enclosed in a vessel at a pressure of 2.5 atm. Due to leak in...
Text Solution
|
- The gas equation PV//T = constant is true for a constant mass of an id...
Text Solution
|
- The density of a certain mass of a gas at STP is d. If the pressure of...
Text Solution
|
- The quantity PV//kT represents
Text Solution
|
- If pressure of a gas contained in a closed vessel is increased by 0.4%...
Text Solution
|
- The pressure P, volume V and temperature T of a gas in the jqar A and ...
Text Solution
|
- A vessel contains 1 mole of O2 gas (relative molar mass 32) at a tempe...
Text Solution
|
- The pressure of a gas kept in an isothermal container is 200Kpa. If ha...
Text Solution
|
- The gas in a vessel is subjected to a pressure of 20 atmosphere at a t...
Text Solution
|
- Two identical cylinders at same temp contains hydrogen at 2.5 atm and ...
Text Solution
|
- A vessel A has volume V and a vessel B has volume 2V. Both contain som...
Text Solution
|
- A flask is filled with 13 g of an ideal gas at 27^(@)C and its tempera...
Text Solution
|
- Air is filled in a bottle and it is corked at 35^(@)C. If the cork can...
Text Solution
|
- Consider the quantity (MkT)/(pV) of an ideal gas where M is the mass o...
Text Solution
|
- Two ballons are filled, one with pure He gas and other by air, repecti...
Text Solution
|
- Two different masses m and 3 m of an ideal gas are heated separately i...
Text Solution
|
- The pressure exterted on the walls of the container by a gas is due to...
Text Solution
|
- At constant volume, temperature is increased. Then
Text Solution
|