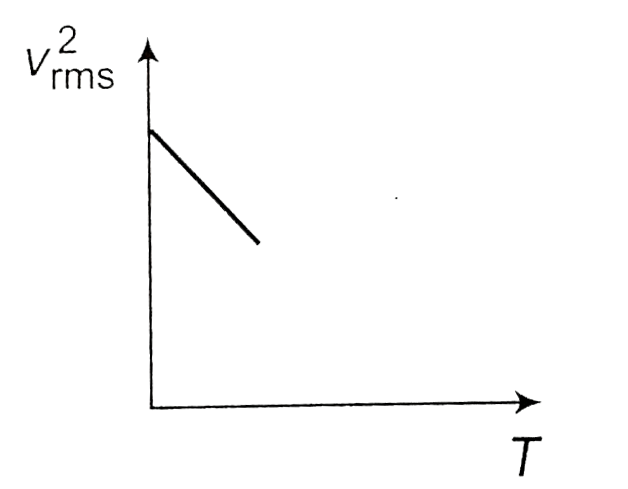A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CP SINGH-KINETIC THEORY OF GASES-Exercises
- Which of the following gases has maximum rms speed at a given temperat...
Text Solution
|
- The temperature at which the rms speed of air molecules is double of t...
Text Solution
|
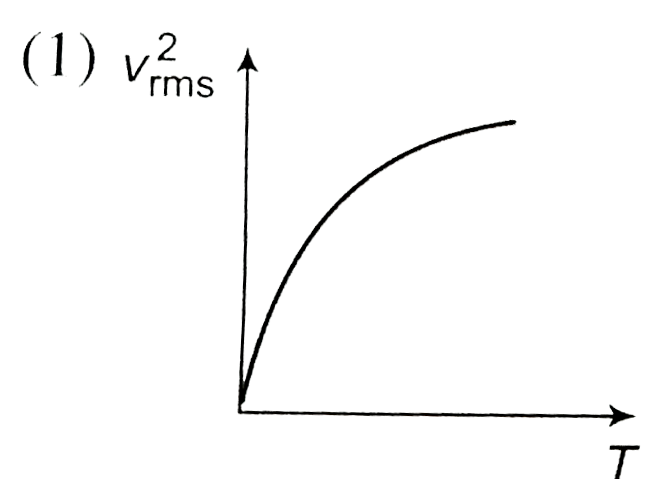
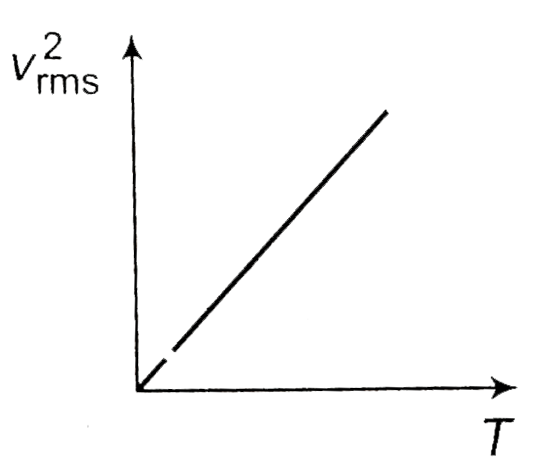
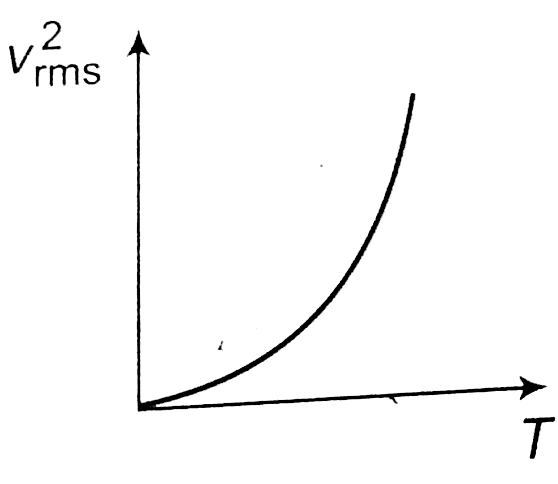
- The curve between absolute temperature and v(rms)^(2) is
Text Solution
|
- The rms speed of oxygen molecules in a gas is v. If the temperature is...
Text Solution
|
- A mixture of 2 moles of helium gas ((atomic mass)=4a.m.u) and 1 mole o...
Text Solution
|
- For gas at a temperature T the root-mean-square speed v(rms), the most...
Text Solution
|
- The root mean square velocity of hydrogen molecules at 300 K is 1930 m...
Text Solution
|
- The root mean square velocity of the molecules in a sample of helium i...
Text Solution
|
- At what temperature is the root mean square velocity of gaseous hydrog...
Text Solution
|
- The molecules of a given mass of gas have a rms velocity of 200 m//sec...
Text Solution
|
- A cubical box with porous walls containing an equal number of O(2) and...
Text Solution
|
- Two vessels have equal volums. One of them contains hydrogen at one at...
Text Solution
|
- The remperature at which the rms speed of hydrogen molecules is equal ...
Text Solution
|
- Suppose a container is evacuated to leave just one molecule of a gas i...
Text Solution
|
- Three closed vessels A, B and C are at the same temperature T and cont...
Text Solution
|
- Let barv,v(rms) and vp respectively denote the mean speed. Root mean s...
Text Solution
|
- The average momentum of a molecule in a sample of an ideal gas depends...
Text Solution
|
- Keeping the number of moles, volume and temperature the same, which of...
Text Solution
|
- At 0 K, which of the following properties of a gas will be zero ?
Text Solution
|
- Which of the following quantities is zero on an average for the molec...
Text Solution
|