Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CP SINGH-HEAT AND CALORIMETRY-Exercise
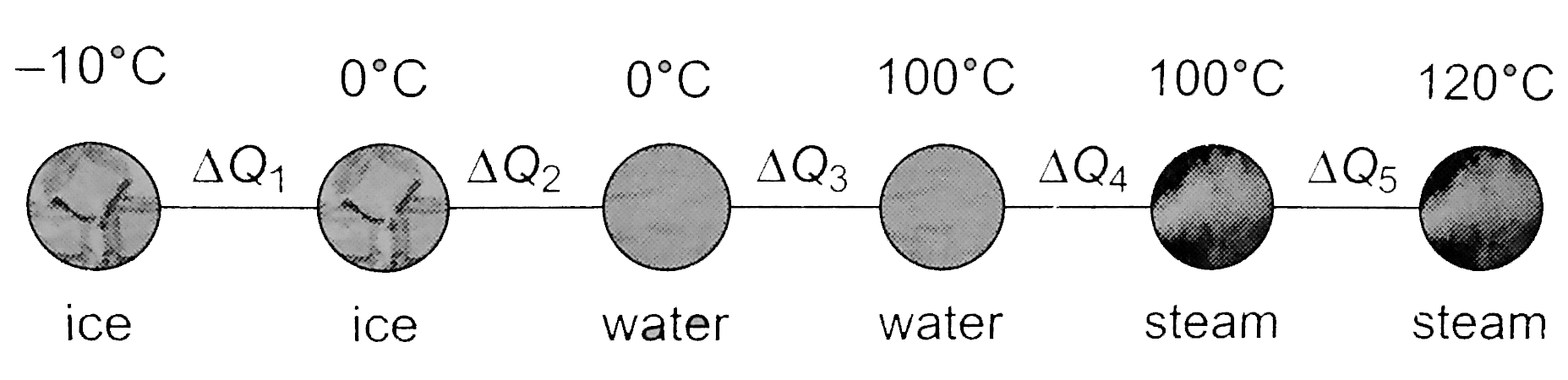
- Calculate the amount of heat required to convert 10 g ice at -10^@C in...
Text Solution
|
- Heat and work are equivalent i.e.,
Text Solution
|
- The temperature of an object is observed to rise in a period. In this ...
Text Solution
|
- If heat is supplied to s solid its temperature (i) must increase (...
Text Solution
|
- The temperature of a solid object is observed to be constant during a ...
Text Solution
|
- If specific heat of a substance is infinite, it means
Text Solution
|
- Boiling water is changing into steam. Under this condition the specifi...
Text Solution
|
- Calorie is defined as the amount of heat required to raise temperature...
Text Solution
|
- A metallic ball and highly stretched spring are made of the same mater...
Text Solution
|
- A 50 kg man is running at a speed of 18 kmH^(-1) If all the kinetic en...
Text Solution
|
- A ball is dropped on a floor from a height of 2.0m. After the collisio...
Text Solution
|
- A lead bullet strikes against a steel plate with a velocity 200ms^-1. ...
Text Solution
|
- A lead bullet penetrates into a solid object and melts. Assuming that ...
Text Solution
|
- An electric kettle takes 4 A current at 220 V. How much time will it t...
Text Solution
|
- Hailstone at 0^@C from a height of 1 km on an insulating surface conve...
Text Solution
|
- Two bodies at different temperature are mixed in a calorimeter.Which o...
Text Solution
|
- A liquid of mass m and specific heat c is heated to a temperature 2T. ...
Text Solution
|
- Two liquids A and B are at 30^@C and 20^@C, respectively When they are...
Text Solution
|
- A vessel contains 110 g of water. The heat capacity of the vessel is e...
Text Solution
|
- The temperature of equal masses of three different liquids A, B and C ...
Text Solution
|
- Heat is supplied to a certain homogeneous sample of matter, at a unifo...
Text Solution
|