A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEAR PHYSICS
NARAYNA|Exercise Previous JEE questions|21 VideosNUCLEAR PHYSICS
NARAYNA|Exercise LEVEL-I-(H.W)|16 VideosNUCLEAR PHYSICS
NARAYNA|Exercise Level-V Multiple answer questions|45 VideosMOVING CHARGES AND MAGNETISM
NARAYNA|Exercise EXERCISE - 4|20 VideosNUCLEI
NARAYNA|Exercise ASSERTION & REASON|5 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-NUCLEAR PHYSICS-NCERT Based question
- Fusion processes, like combining two deuterons to form a He nucleus ar...
Text Solution
|
- Sample of two radioactive nuclides A and B are taken. lambda(A) and la...
Text Solution
|
- The variation of decay rate of two radioactive samples A and B with ti...
Text Solution
|
- He(2)^(3) and He(1)^(3) nuclei have the same mass number. Do they have...
Text Solution
|
- Draw a graph showing the variation of decay rate with number of active...
Text Solution
|
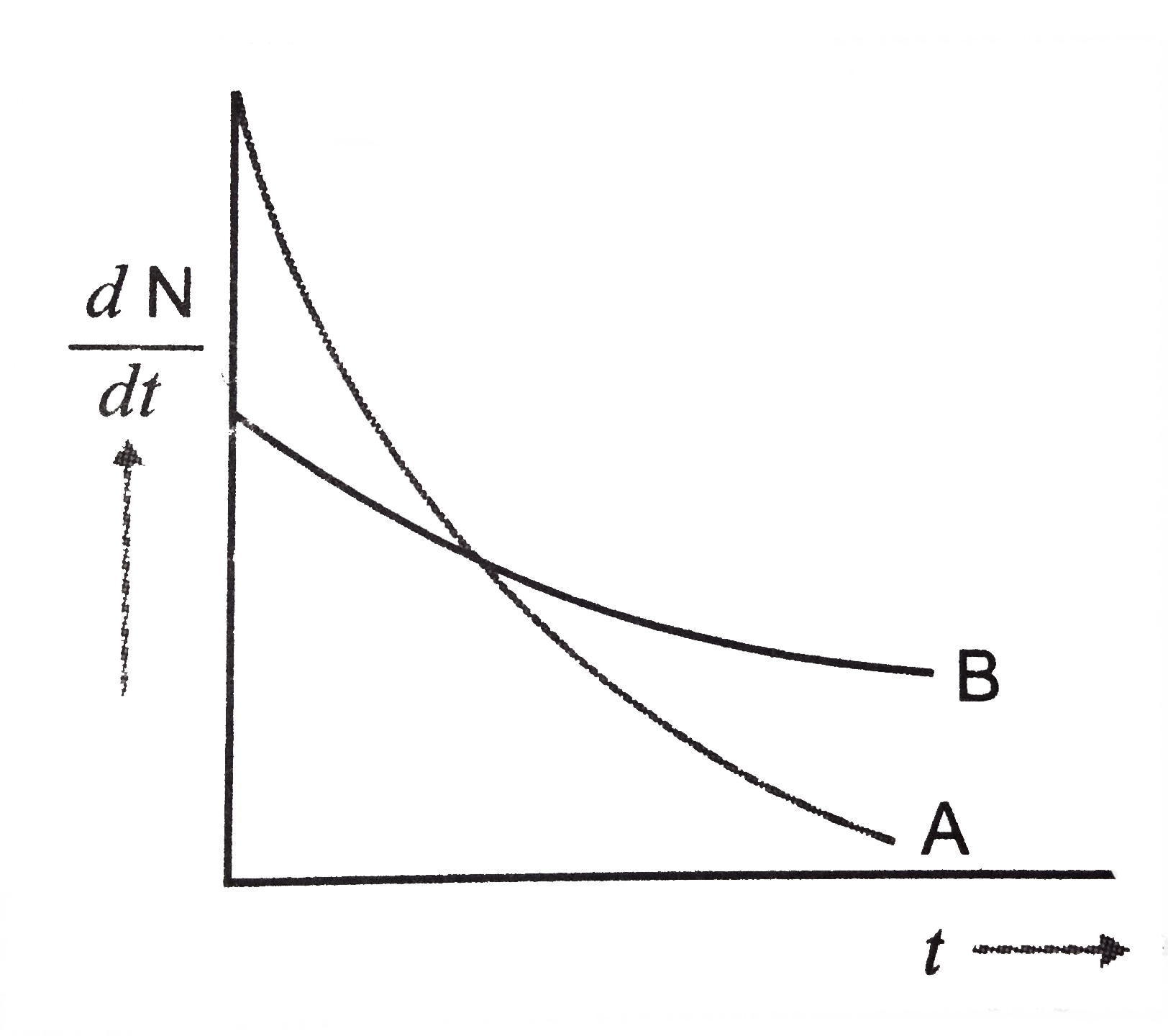
- Which sample A or B shown in figure has shorter mean-life ?
Text Solution
|
- Heavy stable nuclei have more neutrons than protons. This is because o...
Text Solution
|
- Consider a radioactive nucleus A which decays to a stable nucleus C th...
Text Solution
|
- Two radioactive materials X(1) and X(2) have decay constant 11 lambda ...
Text Solution
|
- Radon 220 decays to Bismuth 212 by the following series of decay {:(...
Text Solution
|
- Find the half life of U^(238), if one gram of it emits 1.24xx10^4 alp...
Text Solution
|
- What is the age of an ancient wooden piece if it is known that the spe...
Text Solution
|
- In the uranium ore, the ratio of U^(238) nuclei to Pb^(206) nuclei is ...
Text Solution
|
- The specific activity of a preparation consisting of radioactive Co^(5...
Text Solution
|
- An unstable element is produced in nuclear reaction at a constant rate...
Text Solution
|
- Two identical samples (same material and same amout) P and Q of a radi...
Text Solution
|
- A radionuclide is produced at constant rate 'q' having half life T. Fi...
Text Solution
|
- In an agriculture experiment, a solution containing 1 mole of a radioa...
Text Solution
|
- To investigate the beta-decay of Mg^(23) radionuclide, a counter was a...
Text Solution
|
- Nucleus A decays to B with decay constant lambda(1) and B decays to C ...
Text Solution
|