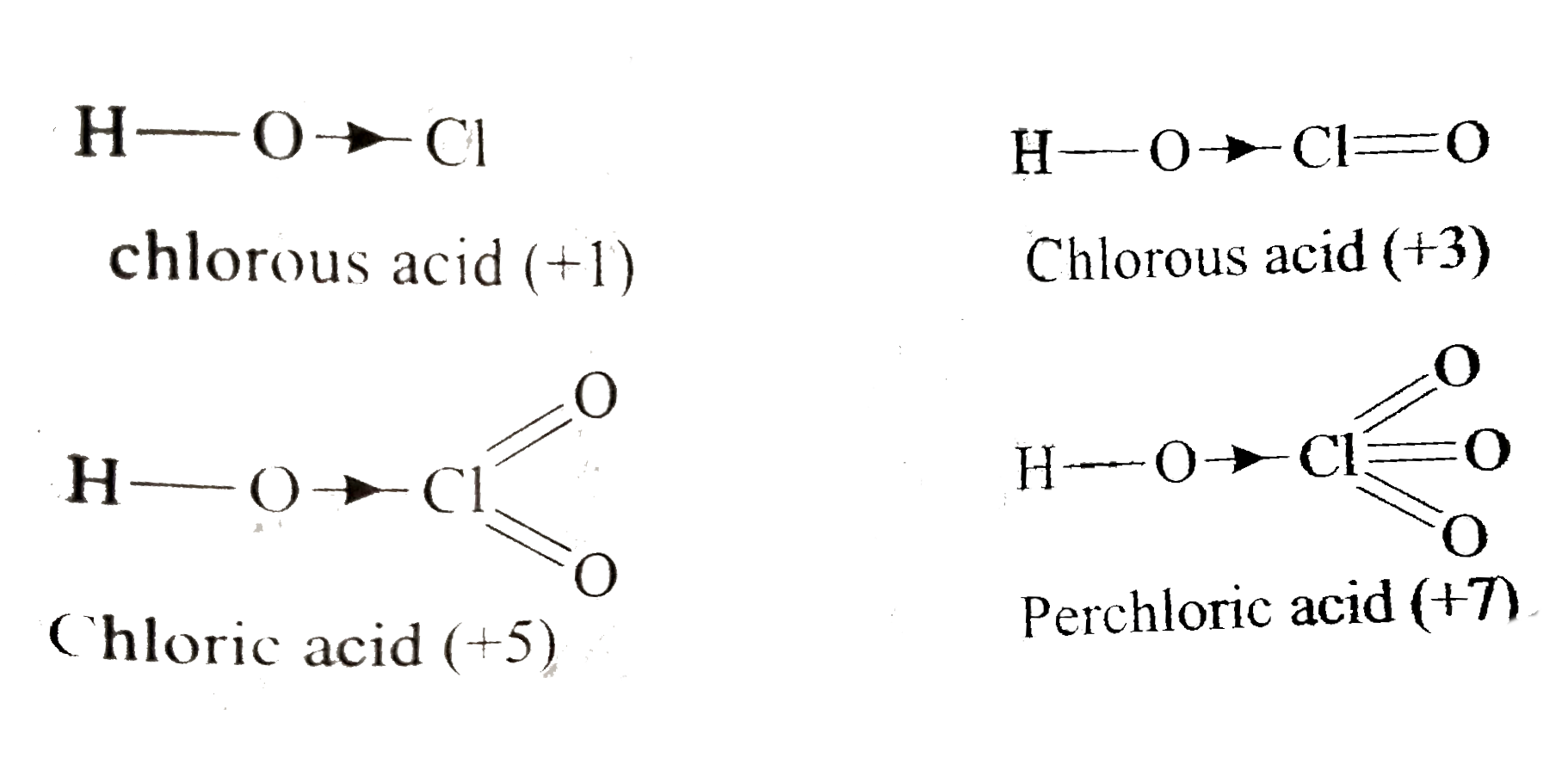A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUILIBRIUM
R SHARMA|Exercise Follow-up Test 12|13 VideosEQUILIBRIUM
R SHARMA|Exercise Follow-up Test 13|10 VideosEQUILIBRIUM
R SHARMA|Exercise Follow-up Test 10|15 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS
R SHARMA|Exercise ARCHIVES|37 VideosGENERAL ORGANIC CHEMISTRY
R SHARMA|Exercise Archives|36 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-EQUILIBRIUM-Follow-up Test 11
- Calculate the concentration of the formate ion present in 0.100 M form...
Text Solution
|
- Which of the following is the weakest acid ?
Text Solution
|
- The correct experssion for Ostwald's dilution law is
Text Solution
|
- The pH of 0.1 M monobasic acid is 4.50. The acidity constant (K(a)) of...
Text Solution
|
- If the concentration of the weak monoprotic acid HA is C mmol L^(-1) ...
Text Solution
|
- Which of the following is the strongest base ?
Text Solution
|
- The pK(b) of NH(3) is 4.75. Calculate the concentration of H^(+) ions ...
Text Solution
|
- K(a) for a weak monobasic acid is 1.0xx10^(-6). The pK(b) of its conju...
Text Solution
|
- If the dissociation constants of two weak acids HA(1) and HA(2) are K(...
Text Solution
|
- Which of the following is arranged in the order of increseing ionizati...
Text Solution
|
- Oxoacids are acids.
Text Solution
|
- Which of the following is correct for a compound of the type ZOH ?
Text Solution
|
- Which of the following oxocids is the strongest acid ?
Text Solution
|
- Which of the following oxoacids is the weakest acid ?
Text Solution
|
- Which of the following order of acidic strengths is incorrect ?
Text Solution
|
- Which of the following is the strongest acid ?
Text Solution
|