A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALCOHOL, PHENOL AND ETHERS-Follow -Up Test -4
- Ethanol has higher boiling point than ethanol because
Text Solution
|
- The hydrogen bonding ability of the isomeric 1^(@),2^(@) and 3^(@) a...
Text Solution
|
- Alcohols have lower boiling points than carboxylic acids of comparable...
Text Solution
|
- The compound which is added as an antifreeze to the water in automobil...
Text Solution
|
- An alcohol , C(3)H(8)O(3) , which is used as a humectant (moistening a...
Text Solution
|
- Which of the following is correct ?
Text Solution
|
- Which of the following statements is incorrect ?
Text Solution
|
- Which of the following statements is not correct ?
Text Solution
|
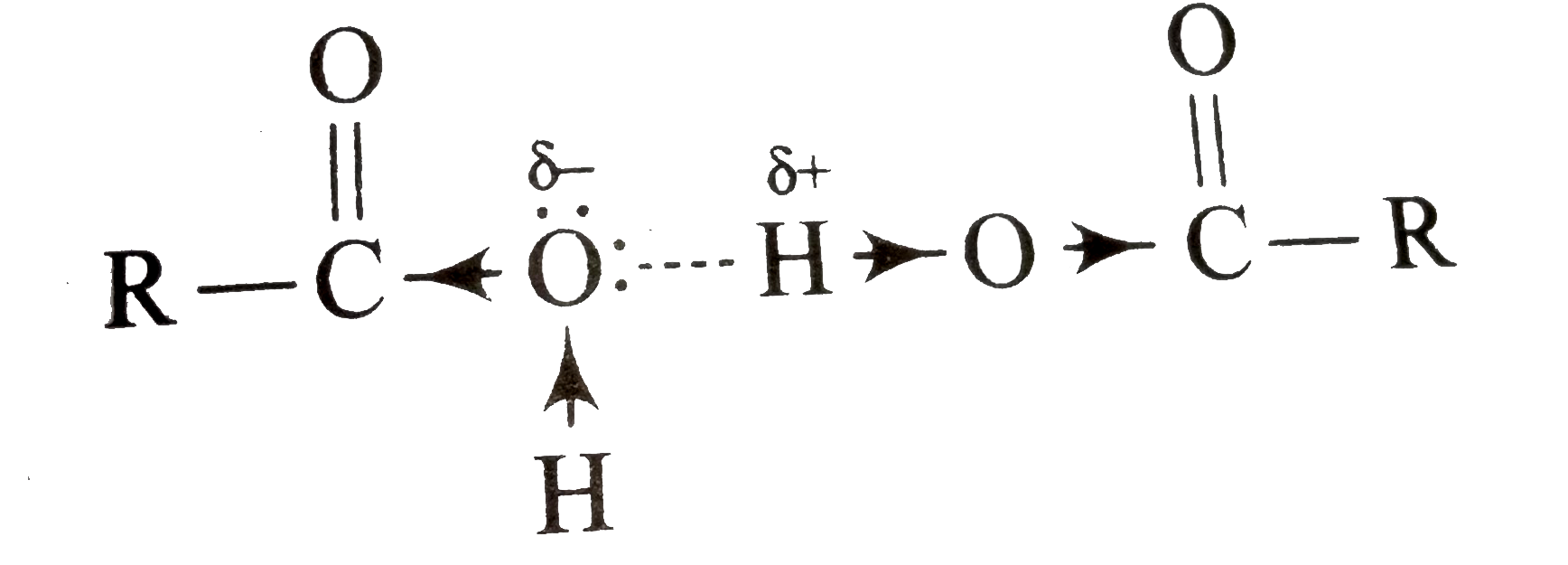 group , the hydrogen (of `OH` groupis carboxylic acid) is more positive to form relatively strong hydrogen bond with oxygen of other molecule.
group , the hydrogen (of `OH` groupis carboxylic acid) is more positive to form relatively strong hydrogen bond with oxygen of other molecule. 