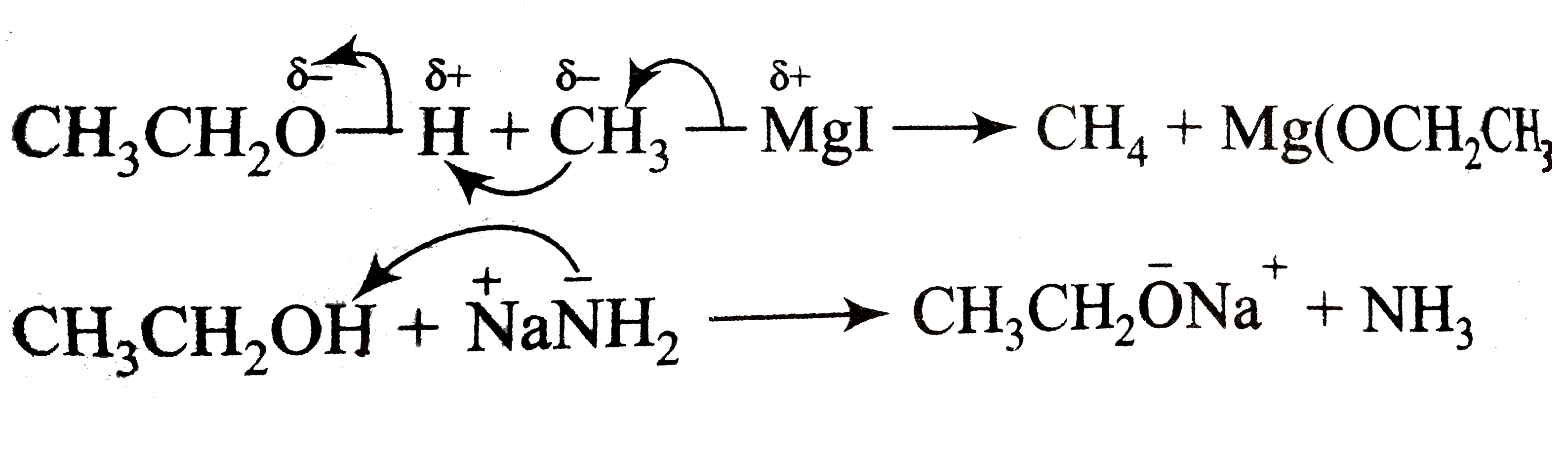A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALCOHOL, PHENOL AND ETHERS-Follow -Up Test -5
- Which of the following compounds on reaction with ethyl alcohol will p...
Text Solution
|
- A compound (X) with the molecular formula C(3)H(8)O can be oxidized to...
Text Solution
|
- An alcohol on vigorous oxidation with K(2)Cr(2)O(7) and H(2)SO(4) give...
Text Solution
|
- Which of the following reagents can distinguish methanol from ethanol ...
Text Solution
|
- Which of the following is the weakest acid?
Text Solution
|
- Which of the following on reaction with aluminimum produces a compound...
Text Solution
|
- Which of the following undergoes exterification with acetic acid at th...
Text Solution
|
- The maximum number of moles of isopropyl alcohol that can be oxidized ...
Text Solution
|
- Which of the following is the most efficient in removing a proton from...
Text Solution
|
- Which of the following reactions reflects the acidic character of etha...
Text Solution
|
- The reagent most suitable for converting a primary alcohol into an ald...
Text Solution
|
- Vapor of t-butyl alcohol is passed over copper heated to 300^(@)C. The...
Text Solution
|
- Which of the following reagent is the most suitable for converting ben...
Text Solution
|
- Which of the following compounds can change the colour of chromic acid...
Text Solution
|