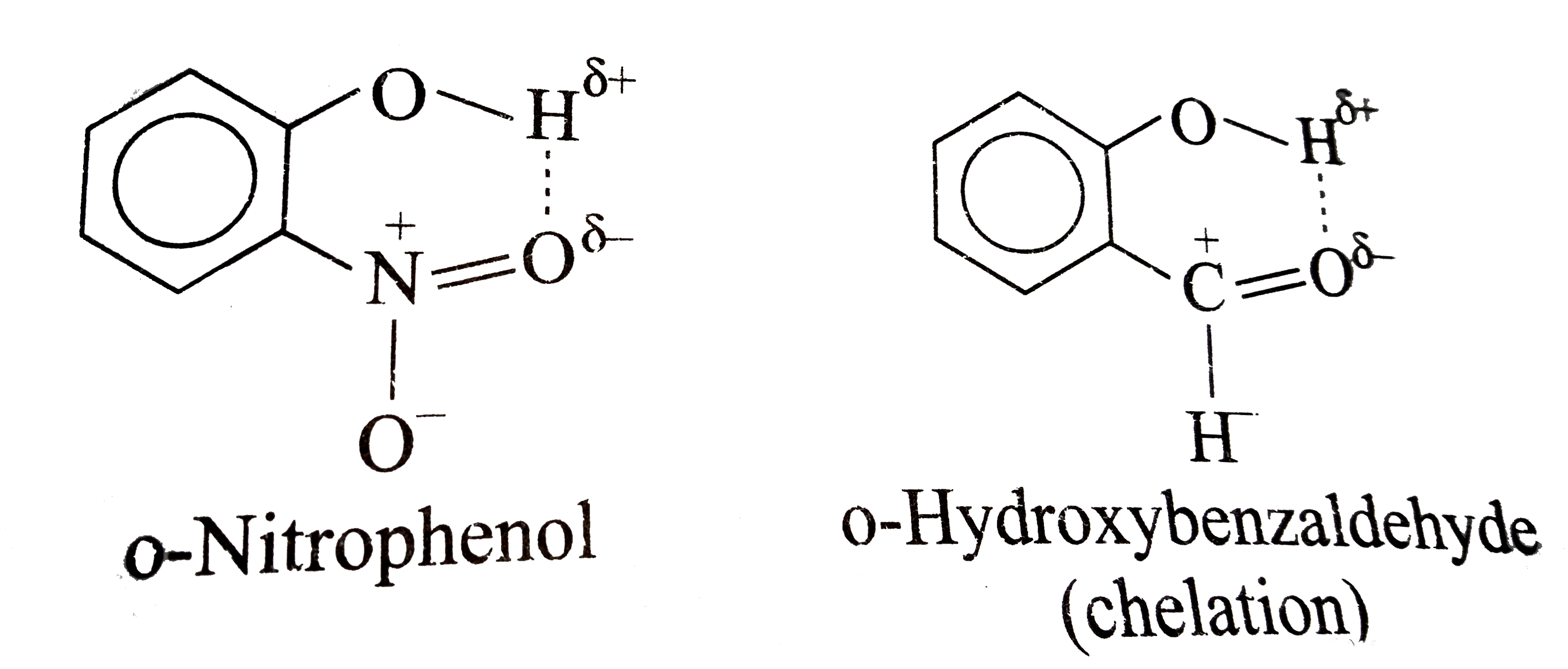A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALCOHOL, PHENOL AND ETHERS-Follow -Up Test -8
- Which of the following are a general group with an OH attached to a ca...
Text Solution
|
- How many tautomeric forms of molecular formula C(6)H(5)OH are possible...
Text Solution
|
- How many phenols of molecular formula C(7)H(8)O are possible ?
Text Solution
|
- Hydroquinone is a ……..phenol
Text Solution
|
- Pyrogallol is
Text Solution
|
- Which of the following statements is not correct ?
Text Solution
|
- Which of the following is non-volatile in steam ?
Text Solution
|
- Which of the following compounds exhibit chelation ? (i) o-cresol ...
Text Solution
|