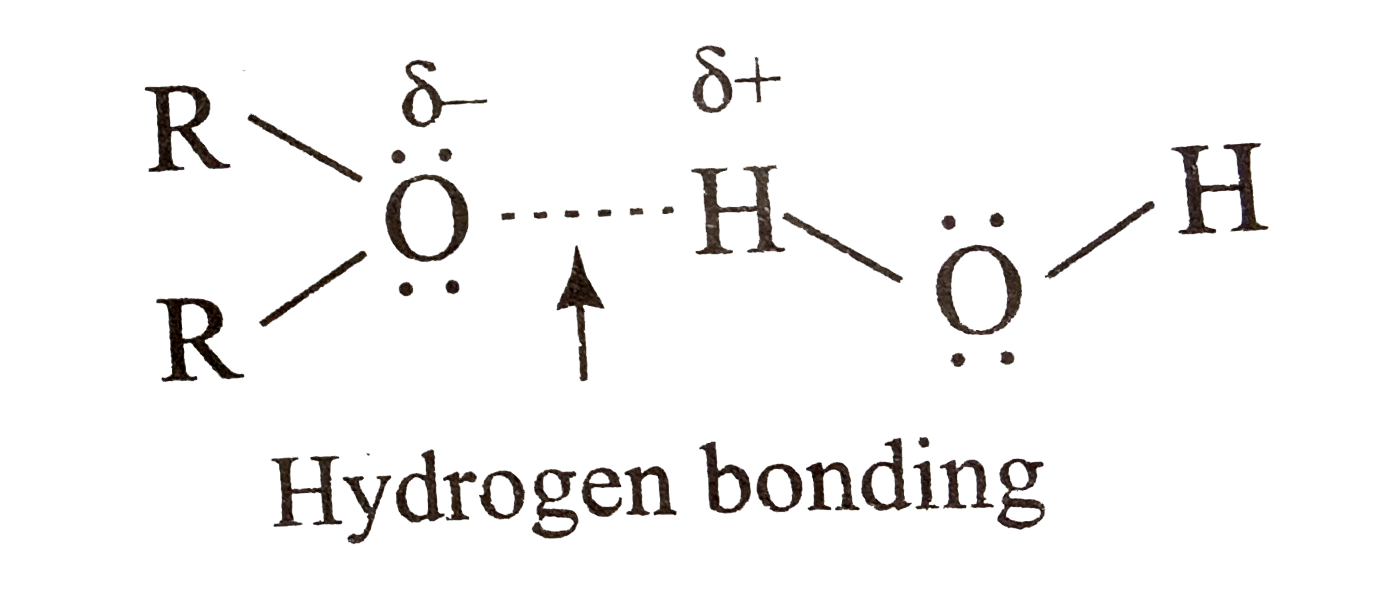A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALCOHOL, PHENOL AND ETHERS-Follow -Up Test -12
- Which of the following pairs have the same general molecular formula C...
Text Solution
|
- Among the following compounds, which one is not an ether?
Text Solution
|
- Which of the following is a constitutionally symmetrical ether?
Text Solution
|
- While of the followig should be considered as an aromatic ether?
Text Solution
|
- The IUPAC name of the compound is
Text Solution
|
- The number of ethers possible with the molecular formula C(4)H(10)O is...
Text Solution
|
- The two structure represent
Text Solution
|
- Th C-O bonds of saturated aliphatic ehters are formed by the linear or...
Text Solution
|
- Which of the following is a cyclic ether possessing the characterstics...
Text Solution
|
- The crow ether is a heterocyclic polyether, usually with at least "" o...
Text Solution
|
- Th C-O-O bond angle in the ether molecule is
Text Solution
|
- Ethers are
Text Solution
|
- Which of the following is incorrect?
Text Solution
|
- CH(3)OCH(3) is a gas at room temperature while its isomer CH(3)CH(2)OH...
Text Solution
|