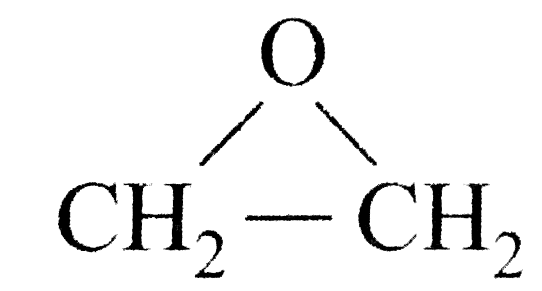
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALCOHOL, PHENOL AND ETHERS
R SHARMA|Exercise Quation Bank(Building the knowledge) Level III|30 VideosALCOHOL, PHENOL AND ETHERS
R SHARMA|Exercise Quation Bank(Building the knowledge) Level IV|14 VideosALCOHOL, PHENOL AND ETHERS
R SHARMA|Exercise Quation Bank(Building the knowledge) Level I|7 VideosAMINES
R SHARMA|Exercise Archives|36 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-ALCOHOL, PHENOL AND ETHERS-Quation Bank(Building the knowledge) Level II
- Which of the following is commonly used in anhydrous condition for the...
Text Solution
|
- A compound with the formula C(4)H(10)O yields another compound C(4)H(8...
Text Solution
|
- Which of the following will form a yellow precipitate of iodoform on h...
Text Solution
|
- CH(3)CH=CHCH(OH)CH(3)rarrCH(3)CH=CHCOCH(3) which of the following reag...
Text Solution
|
- In CH(3)CH(2)OH, the bond that undergoes heterolytic cleavage most rea...
Text Solution
|
- Among the following alcohols, which has the highest solubility in wate...
Text Solution
|
- Which of the following reagents can distinguish between t-butyl alcoho...
Text Solution
|
- underset(CH(2)OH)overset(CH(2)OH)(" | ") overset(PBr(3))(rarr)...
Text Solution
|
- Identify the products in the following reaction 3CH(3)CH=CH(2)overse...
Text Solution
|
- HBr reacts fastest with
Text Solution
|
- Ethanol and dimethyl ether form a pair of of functional isomers. The b...
Text Solution
|
- In the reaction given below, X is C(6)H(5)MgBr+CH(3)OHrarrX
Text Solution
|
- In the reaction given below, X is Neopentyl alcohol overset(H(2)SO(4...
Text Solution
|
- Lucas test is done for
Text Solution
|
- When alcohol reacts with concentrated H(2)SO(4), the intermediate spec...
Text Solution
|
- The only alcohol that can be prepared by the direct hydration of alken...
Text Solution
|
- Which of the following alkenes will give optically active alcohol when...
Text Solution
|
- The most suitable reagent for the conversion of RCH(2)OHrarrRCHO is
Text Solution
|
- 1-phenylethanol can be prepared by reaction of benzaldehyde with
Text Solution
|
- In the reaction sequence CH(2)=CH(2) overset("hydrochlorous")underse...
Text Solution
|