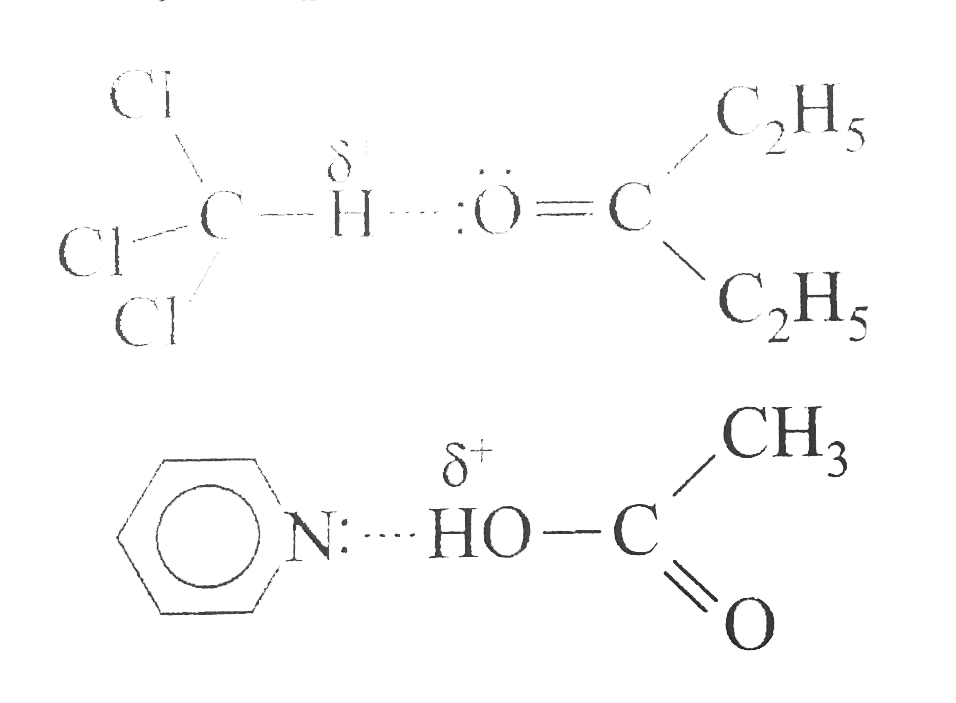
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-SOLUTIONS-FOLLOW-UP TEST 10
- Which of the following conditions is correct for an ideal solution?
Text Solution
|
- Positive deviation from Raoult's law are exhibited by binary liquid mi...
Text Solution
|
- Which of the following paris will show a negative deviation from Raoul...
Text Solution
|
- Which one of the following is not an ideal solution?
Text Solution
|
- An azeotropic solution of two liquids has a boiling point higher than ...
Text Solution
|
- An azeotropic mixture of HCl and water has
Text Solution
|
- Pure water boils at 373.15 K and nitric acid boils at 359.15 K. An aze...
Text Solution
|