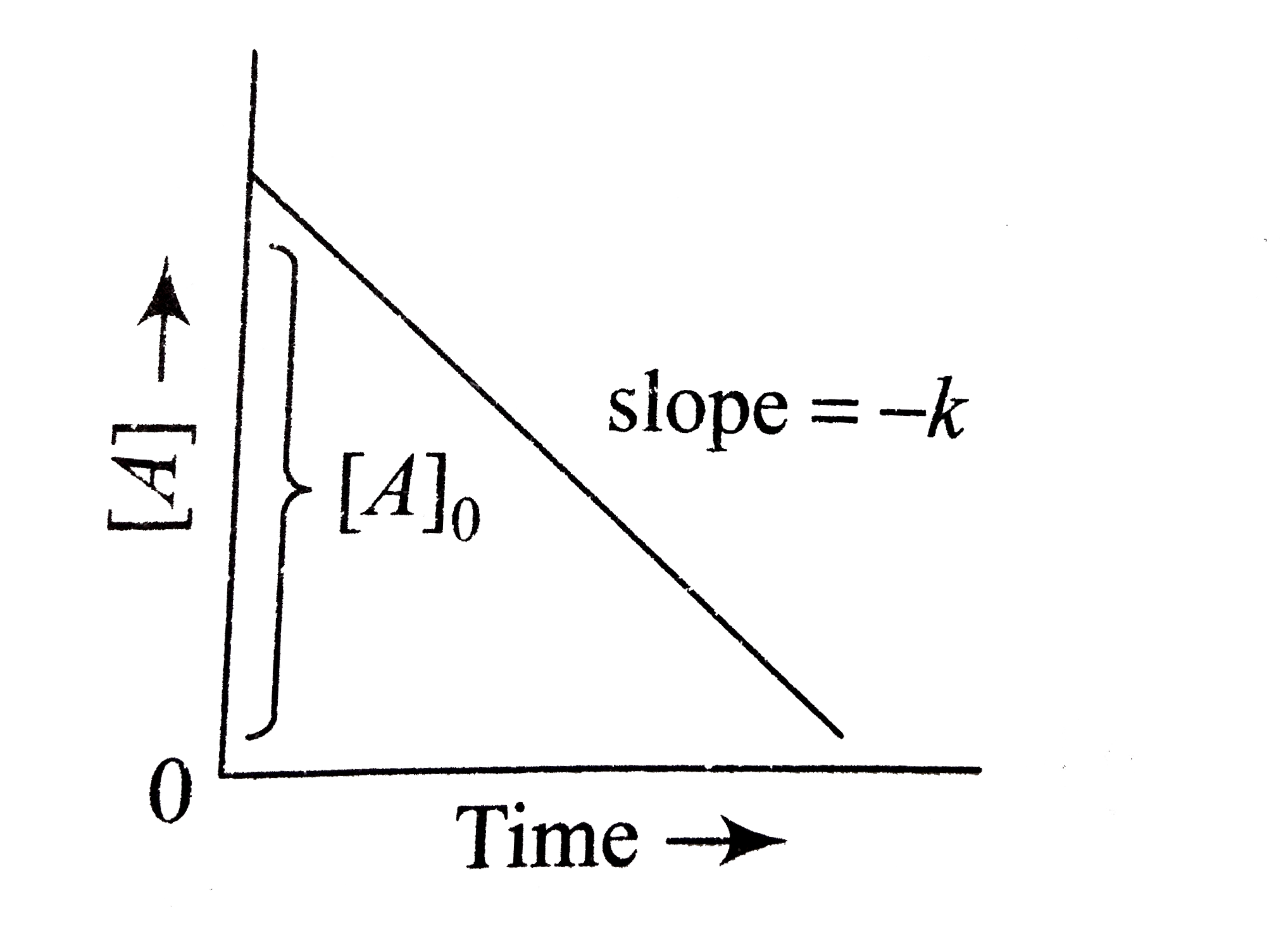
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL KINETICS-Follow-up 4
- For a zero order reaction of the type A rarr products, the integrated ...
Text Solution
|
- Reactant 'A' (initial concentration, a) reacts according to zero order...
Text Solution
|
- The reaction A(g)rarrB(g) is found to be zero order with respect to A(...
Text Solution
|
- Which of the following graphs is the characteristic of a zero order r...
Text Solution
|
- How many half-lives are needed to complete the zeroth order reaction ?
Text Solution
|
- In a certain reaction, 10% of the reactant decomposes in one hour, 20%...
Text Solution
|
- For a first order reaction. When the initial concentration, a is not k...
Text Solution
|
- The reaction 2N(2)O(5)(g)rarr4NO(2)(g)+O(2)(g) is found to be firt...
Text Solution
|
- The concept of half life is useful for the reactions of
Text Solution
|
- The integrated rate expression for a first order reaction can be writt...
Text Solution
|
- Which of the following curves represents a first order reaction.
Text Solution
|
- For a first order reaction
Text Solution
|
- Which of the following curves represents a second order reaction ? [x ...
Text Solution
|
- A gaseous substance (AB(3)) decomposes according to the overall equati...
Text Solution
|
- The time required for the decompoistion of 99.9% fraction of a first o...
Text Solution
|
- At 373 k, the following reaction A(g)rarr2B(g)+C(g) is found to be of...
Text Solution
|
- The rate law of the reaction A+2Brarr Product is given by (d("Product"...
Text Solution
|
- Which of the following is an example of a pseudop first order reaction...
Text Solution
|
- Rate for a zero order reaction is 2xx10^(-2) mol L^(-1) s^(-1) . If th...
Text Solution
|
- Which of the following curves represents a third order reaction ?
Text Solution
|