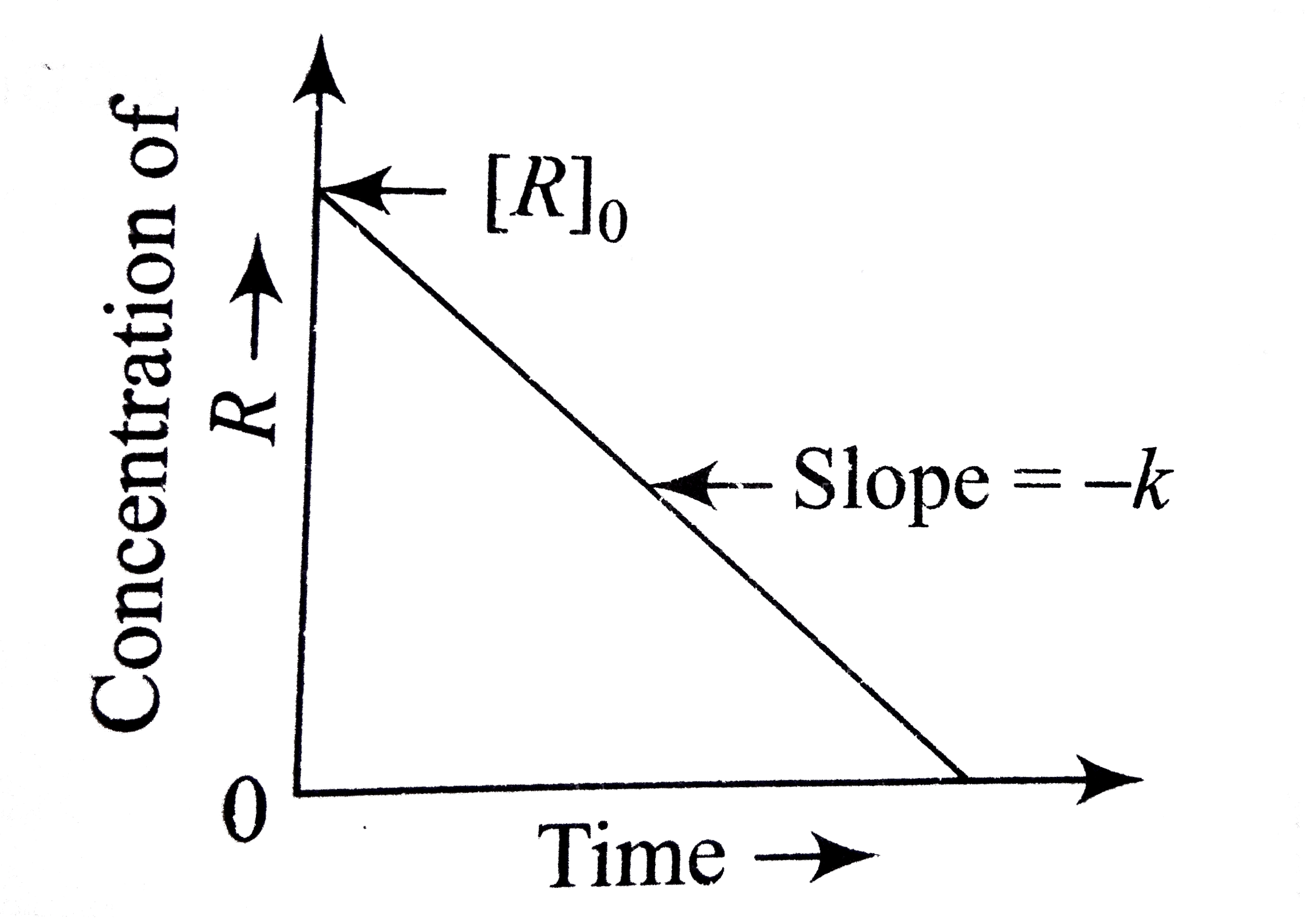
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL KINETICS-Question Bank (Building the knowledge)
- The experiment data for the reaction 2A + B(2) rarr 2AB is |{:("Expe...
Text Solution
|
- The activation energy for a simple chemical reaction A rarr B is E(a) ...
Text Solution
|
- The plot of concentration of the reactant vs time for a reaction is a ...
Text Solution
|
- A chemical reaction has catalyst X. Hence X
Text Solution
|
- For an exothermic reaction, the energy of activation of the reactants ...
Text Solution
|
- Higher order (gt3) reaction are rare due to :
Text Solution
|
- The rate of a reaction doubles when its temperature changes form 300 K...
Text Solution
|
- Plots showing the variation of the rate constant (k) with temperature ...
Text Solution
|
- For a first order reaction A rarr P, the temperature (T) dependent rat...
Text Solution
|
- Conisder a reaction aG+bH rarr Products. When concentration of both th...
Text Solution
|
- The time for half-life period of a certain reaction, A rarr products i...
Text Solution
|
- Rate of a reaction can be expressed by Arrhenius equation as: k = Ae...
Text Solution
|
- The reaction M(2)(g) rarrN(g)+(1)/(2)R(g) shows increase in pressure f...
Text Solution
|
- The raction 2FeCl(3) + SnCl(2) rarr 2FeCl(2) + SnCl(4) is an example ...
Text Solution
|
- A first order gas reaction has k = 1.5 xx 10^(-6) s^(-1) at 200^(@)C. ...
Text Solution
|
- While studying the decompoistion of gaseous N(2)O(5), it is observed t...
Text Solution
|
- 99% at a first order reaction was completed in 32 min. When will 99.9%...
Text Solution
|
- If the rate constant for a reaction repreasented by 2HI rarr H(2)+I(2)...
Text Solution
|
- In an exothermic reaction A rarr B, the activation energy of the forwa...
Text Solution
|
- In Arrhenius equation k = Ae^(-E(a)//RT)), A is the value of the rate ...
Text Solution
|