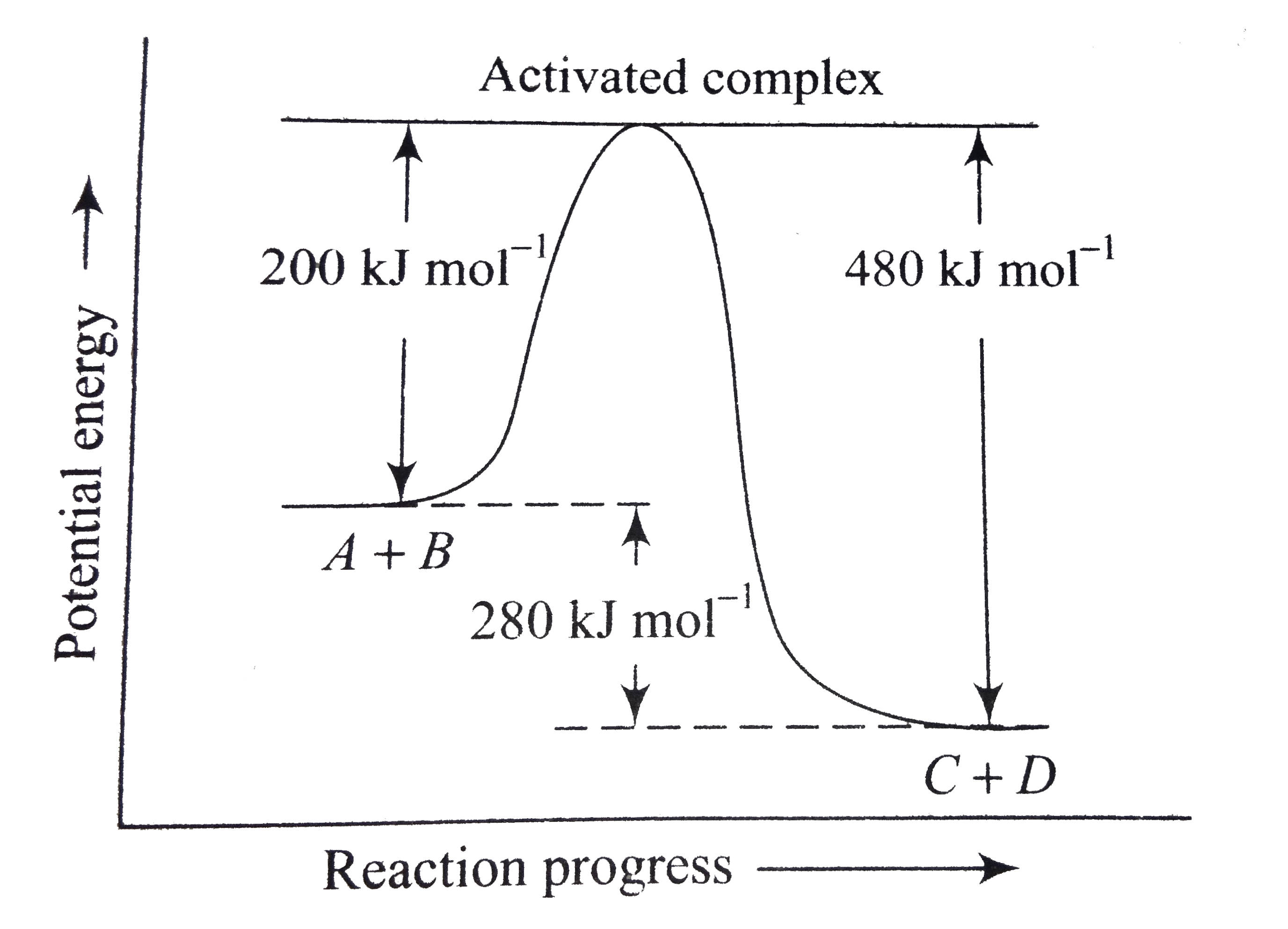
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL KINETICS-Question Bank (Building the knowledge)
- 99% at a first order reaction was completed in 32 min. When will 99.9%...
Text Solution
|
- If the rate constant for a reaction repreasented by 2HI rarr H(2)+I(2)...
Text Solution
|
- In an exothermic reaction A rarr B, the activation energy of the forwa...
Text Solution
|
- In Arrhenius equation k = Ae^(-E(a)//RT)), A is the value of the rate ...
Text Solution
|
- Two reaction proceed at 35^(@)C at the same rate. The temperature coef...
Text Solution
|
- A substance 'A' decomposes by a first order reaction starting initial...
Text Solution
|
- The concentration of R in the reaction R rarr P was measured as a func...
Text Solution
|
- Under the same reaction conditions, the intial concentration of 1.386 ...
Text Solution
|
- Consider a reaction, 2A + B rarr Products When concentration of B al...
Text Solution
|
- The energies of activation for forward and reverse reaction for A(2)+...
Text Solution
|
- NH(3) gaws is adsorbed on the metal surface like tungsten This follows...
Text Solution
|
- The rate law for a reaction between A and B is given by rate = k[A]^(n...
Text Solution
|
- In a first order reaction, the concentration of the reactant decreases...
Text Solution
|
- The hydrogenation of vegetable ghee at 25^(@)C reduces the pressure of...
Text Solution
|
- t some temperature, the rate constant for the reaction of the type ...
Text Solution
|
- For first order reaction involving 2A rarr products the specific react...
Text Solution
|
- The rate of first order reaction is 0.04 mol L^(-1) s^(-1) at 10 min a...
Text Solution
|
- The rate expression for an nth - order reaction (n != 1) is
Text Solution
|
- The general expression for half-life period of an nth order reaction (...
Text Solution
|
- In the reaction, P+Q rarr R+S the time taken for 75% reaction of P i...
Text Solution
|