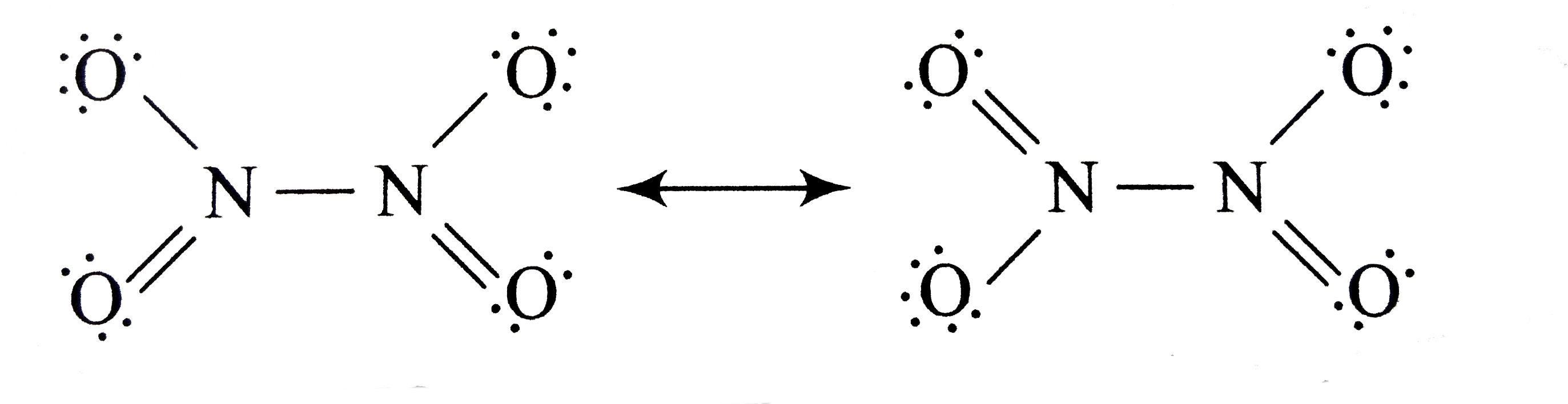
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-THE P BLOCK ELEMENTS-Follow-up Test 6
- Which of the following is wrong for nitrogen (I) oxide?
Text Solution
|
- Nitrous oxide reacts with red -hot copper to give
Text Solution
|
- Sodium azide (NaN(3))is prepared by heating a mixture of
Text Solution
|
- In the N(2)Omolecule the
Text Solution
|
- Nitric oxide(NO)is prepared in the laboratory by
Text Solution
|
- Which of the following statement is incorrect for nitric oxide (NO)?
Text Solution
|
- The number of valence electrons in nitric oxide is11. Among these,one ...
Text Solution
|
- The resonating structure of NO are represented by
Text Solution
|
- Which of the following complexes is responsible for the brown colour o...
Text Solution
|
- Which of the following statement is correct regarding the nitric oxide...
Text Solution
|
- Nitrogen sesquoxide (N(2)O(3))canbe obtained by cooling (below-30^(@)C...
Text Solution
|
- Nitrogen sesquoxide (N(2)O(3) is a//an
Text Solution
|
- NO(2) a red -brown poisonous gas is prepared in the laboratory by heat...
Text Solution
|
- which of the following statement is not correct for the nitrogen dioxi...
Text Solution
|
- In the formation of the dimer N(2)O(4)from two molecules of NO(2)the o...
Text Solution
|
- NO(2) reacts with F(2) to give
Text Solution
|
- Liquid N(2)O(4)is a useful nonaqueous solvent in which
Text Solution
|
- Dinitrogen pentoxide ,a colourless deliquescentsolid is ,prepared by
Text Solution
|
- Which of the following statement is correct?
Text Solution
|
- Which of the following is incorrect for nitrogen pentoxide?
Text Solution
|