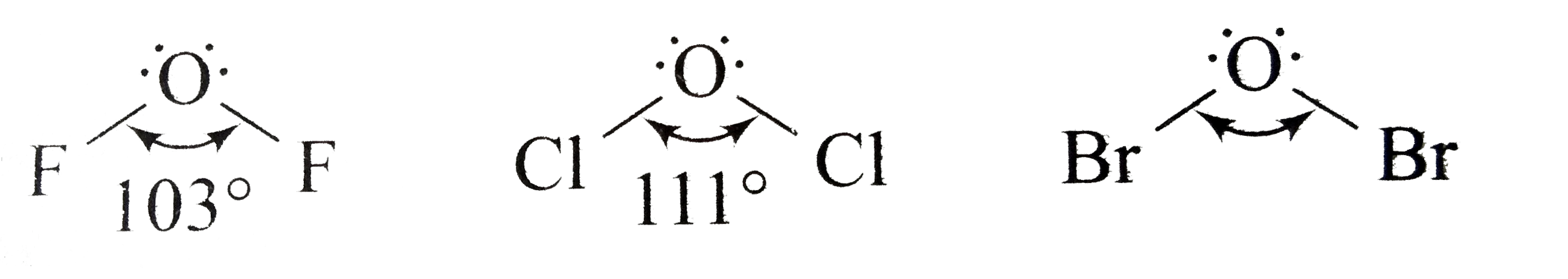A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-THE P BLOCK ELEMENTS-Follow-up Test 16
- Which of ht efollowing is wrong for hydrogen fluoride?
Text Solution
|
- Which of the following acids is used to manufactrure glass shell of te...
Text Solution
|
- Which of the following acids is usually used for the preparation of HB...
Text Solution
|
- Which of the following order is incorrect?
Text Solution
|
- Oxygen difluoride(OF), a pale yellow gas ,is prepared by passing F(2) ...
Text Solution
|
- Dichlorine monoxide (Cl(2)O) a yellow -brown gas ,dissolves inNaOHsolu...
Text Solution
|
- Dichlorine monoxide (Cl(2)O) is prepared by heating freshly precipitat...
Text Solution
|
- Chlorine dioxide reacts with ozone to yield
Text Solution
|
- Which of the following chemical reaction is the safest laboratory prep...
Text Solution
|
- Which of the following is arranged inorderof increasing bond angle?
Text Solution
|