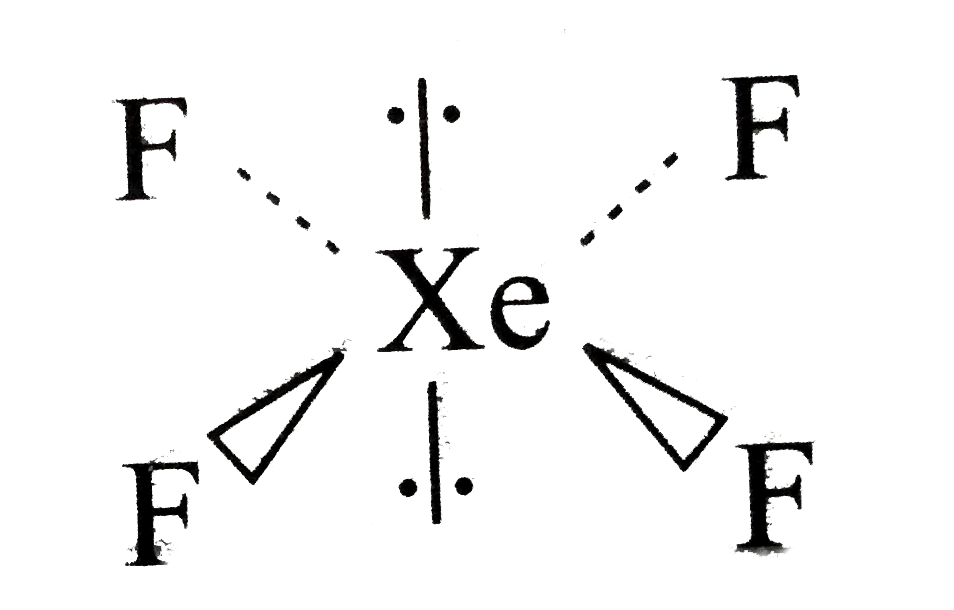A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-THE P BLOCK ELEMENTS-Follow-up Test 20
- Which of the follwing molecular species does not exist?
Text Solution
|
- Which of the following noble gases does not form clathrate compounds?
Text Solution
|
- Which of the following noble gases has the first ionization enthalpy w...
Text Solution
|
- The interaction of Xe and PtF(6) gives a mixture of compounds that con...
Text Solution
|
- Xenon tetrafluoride is prepared by heating Xe and F(2) at 873K,7 bar i...
Text Solution
|
- Xenon fluorides are all extermely strong
Text Solution
|
- Which of the xenon fluorides undergoes disproportionation during the h...
Text Solution
|
- Which of the following compounds is an explosive white hygroscopic sol...
Text Solution
|
- Which of the following compounds of xenon is nonpolar?
Text Solution
|
- For breathing. Deep-sea divers use a mixture of dioxygen and
Text Solution
|
- which of the following nobel gases is used in shop signs and street la...
Text Solution
|