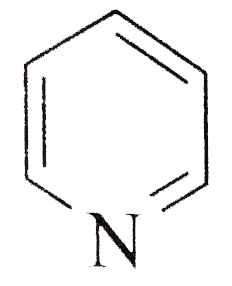
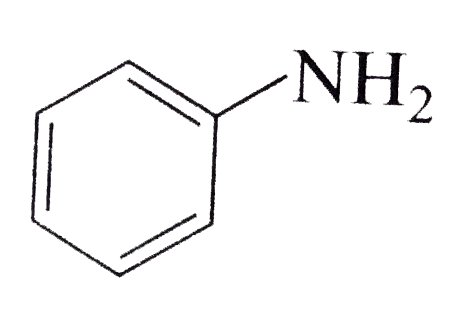
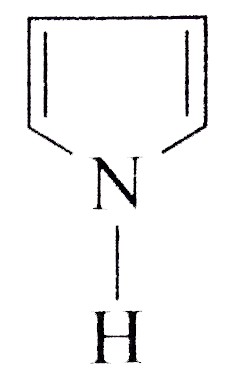A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-AMINES-LevelII
- Which of the following compounds possesses two configurationally stabl...
Text Solution
|
- When stannous chloride and hydrochloric acid are used as the reducing ...
Text Solution
|
- The reation of RCONH(2) with a mixture of Br(2) and aqueous KOH gives ...
Text Solution
|
- Consider the following sequence of reactions The product t(C ) cont...
Text Solution
|
- Consider the following sequence of reactions The final product (B) is ...
Text Solution
|
- Consider the following sequence of reactions (CH(3))(2)CHCOOHunderse...
Text Solution
|
- Which of the following is least reactive toward complex formation with...
Text Solution
|
- The product formed in the reaction .
Text Solution
|
- Diazonium salts form phenylhydrazines when reduced with zinc and hyd...
Text Solution
|
- In the reaction shown below the major product (s) formed is/are .
Text Solution
|
- In the above reaction the product is
Text Solution
|
- (i) red hot iron 873K(ii) fuming HNO(3) H(2)SO(4) heat (iii) H(2)S.NH(...
Text Solution
|
- In the following reactions the product S is
Text Solution
|
- In the following reactions the product S is
Text Solution
|
- Treatment of cyclobutylmethyamine with nitrous acid does not give .
Text Solution
|
- Pyridine is less basic than triethylamine because .
Text Solution
|
- Which of the following chemicals are used to manufacture methy1 isocya...
Text Solution
|
- The strongest base among the following .
Text Solution
|
- Among the following dissocitation constant is highest for .
Text Solution
|
- Coupling of diazonium salts of following takes place in the order
Text Solution
|