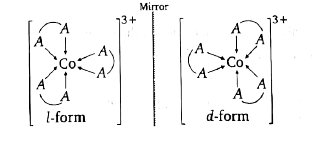
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL-CO-ORDINATION COMPOUNDS-ASSERTION-REASON TYPE QUESTIONS
- Which of the following compound show optical isomerism?
Text Solution
|
- Assertion: In N(2) Molecule, any N-atom can coordinate with central at...
Text Solution
|
- Assertion: In N(2)H(4), any one N-atom can coordination with central m...
Text Solution
|
- These questions consist of two statements each, printed as Assertion a...
Text Solution
|
- Assertion: Acidified [Cu(NH(3))(4)]^(2+) and [Cu(H(2)O)(4)]^(2+) both ...
Text Solution
|
- Assertion: [Fe(EDTA)]^(-) complex is octahedral in shape. Reason: ED...
Text Solution
|
- STATEMENT-1: Tetrahedral complexes with chiral structure exhibit optic...
Text Solution
|
- Assertion: Oxidation state of Fe in Fe(CO)(5) is zero. Reason: Syner...
Text Solution
|
- Assertion: Zeise's salt is a pi-bonded organometallic compound. Reas...
Text Solution
|
- Assertion: [ClCl(3)(NH(3))(3)] does not give white precipitate with Ag...
Text Solution
|
- Assertion: Transition metal ion forming octahedral complexes undergo s...
Text Solution
|
- Assertion: Complex ion [Co(NH(3))(6)]^(2+) is readily oxidized to [Co(...
Text Solution
|
- Assertion: Hydrazine is a neutral ligand. Reason: It has two N as do...
Text Solution
|
- Reason: Complex anion [Re(2)Cl(8)]^(2-) has one delta-bond, one sigma ...
Text Solution
|
- Assertion: In N(2) Molecule, any N-atom can coordinate with central at...
Text Solution
|
- Assertion: In N(2)H(4), any one N-atom can coordination with central m...
Text Solution
|
- Assertion: [Ti(H(2)O)(6)]^(4+) is coloured while [Sc(H(2)O)(6)]^(3+) i...
Text Solution
|
- Assertion: Acidified [Cu(NH(3))(4)]^(2+) and [Cu(H(2)O)(4)]^(2+) both ...
Text Solution
|
- Assertion: [Fe(EDTA)]^(-) complex is octahedral in shape. Reason: ED...
Text Solution
|
- Assertion: Tetrahedral complexes with chiral structure exhibit optical...
Text Solution
|
- Assertion: Oxidation state of Fe in Fe(CO)(5) is zero. Reason: Syner...
Text Solution
|