A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NIKITA PUBLICATION-ATOMS, MOLECULES AND NUCLEI-MCQs
- After an interval of one day , 1//16th initial amount of a radioactiv...
Text Solution
|
- The half - life of ^(215) At is 100 mu , s.The time taken for the radi...
Text Solution
|
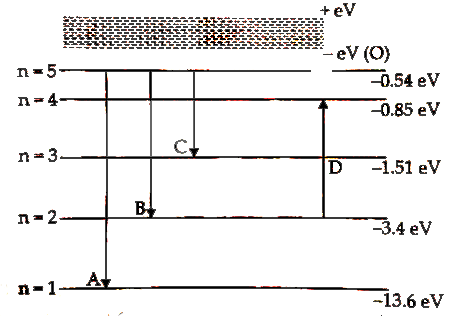
- Inm the figure, energy levels of hydrogen atom have been shown along w...
Text Solution
|
- The radius of the nucleus .(8)O^(16) is 3xx10^(-15)m. The density of t...
Text Solution
|
- Which of the following is stable?
Text Solution
|
- The radius of nucleus is:
Text Solution
|
- Charge on an alpha-particle is
Text Solution
|
- The radius of hydrogen atom, in its ground state, is of the order of
Text Solution
|
- The ionization potential of a hydrogen atom is 13.6 eV. What will be t...
Text Solution
|
- If a radioactive element is placed in an evacuated container, its rate...
Text Solution
|
- The ratio of the speed of the electron in the first Bohr orbit of hydr...
Text Solution
|
- unit of 'lambda' in radioactivity is
Text Solution
|
- The gamma radiations are
Text Solution
|
- What is the amount of energy released when 3 kg mass is annihilated?
Text Solution
|
- The shortest wavelength for Lyman series is 912 Å. What will be the lo...
Text Solution
|
- Balmer series lies in which spectrum?
Text Solution
|
- Balmer series is obtained in
Text Solution
|
- lambda(a)//lambda(beta) in Balmer series is
Text Solution
|
- An electron in first orbit of hydrogen moves in circular orbit of radi...
Text Solution
|
- The electron in the first orbit of hydrogen has velocity 2.18xx10^(6) ...
Text Solution
|