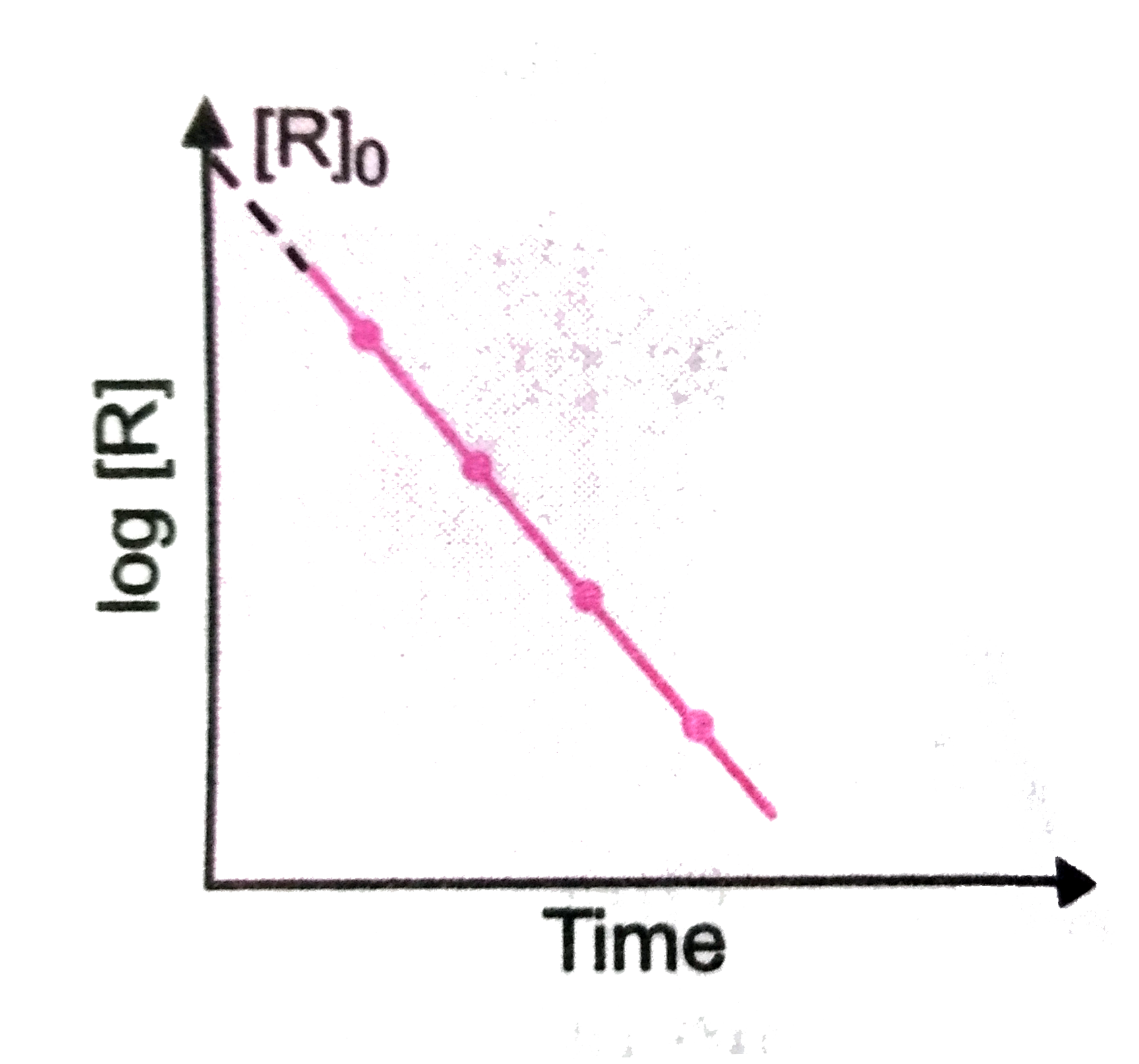
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS
PRADEEP|Exercise CONCEPTUAL QUESTIONS (V. First order reactions, pseudo first order reactions and radioactive disintegration)|3 VideosCHEMICAL KINETICS
PRADEEP|Exercise CONCEPTUAL QUESTIONS (VI. Activation energy, effect of temp. on rate (Arrhenius eqn.) and effect of catalyst on rate)|9 VideosCHEMICAL KINETICS
PRADEEP|Exercise CONCEPTUAL QUESTIONS (III. Molecularity of a reaction, its mechanism and difference between order and molecularity)|2 VideosBIOMOLECULES
PRADEEP|Exercise IMPORTANT QUESTIONS (FOR BOARD EXAMINATION)|25 VideosCHEMISTRY IN EVERYDAY LIFE
PRADEEP|Exercise IMPORTANT QUESTION FOR BOARD EXAMINATION|30 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL KINETICS-CONCEPTUAL QUESTIONS (IV. Integrated rate equations, half-life, determination of rate law, rate constant and order)
- For a certain chemical reaction, variation in the concentration in [R]...
Text Solution
|
- Consider the reaction Aoverset(K)to P. The change in concentration of ...
Text Solution
|
- Observe the graph in the diagram and answer the following questions : ...
Text Solution
|
- For a first order reaction, time taken for half of the reaction to com...
Text Solution
|
- A reaction is 50% complete in 2 hours and 75% complete in 4 hours. Wha...
Text Solution
|
- For a reaction : 2NH(3)(g) overset(Pt)rarr N(2)(g)+3H(2)(g) Rate =...
Text Solution
|